Chronic Ethanol Exposure Potentiates Cholinergic Neurotransmission in the Basolateral Amygdala
- PMID: 33385490
- PMCID: PMC7856184
- DOI: 10.1016/j.neuroscience.2020.12.014
Chronic Ethanol Exposure Potentiates Cholinergic Neurotransmission in the Basolateral Amygdala
Abstract
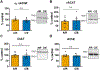
Chronic intermittent ethanol (CIE) exposure dysregulates glutamatergic and GABAergic neurotransmission, facilitating basolateral amygdala (BLA) pyramidal neuron hyperexcitability and the expression of anxiety during withdrawal. It is unknown whether ethanol-induced alterations in nucleus basalis magnocellularis (NBM) cholinergic projections to the BLA mediate anxiety-related behaviors through direct modulation of GABA and glutamate afferents. Following 10 days of CIE exposure and 24 h of withdrawal, we recorded GABAergic and glutamatergic synaptic responses in BLA pyramidal neurons with electrophysiology, assessed total protein expression of cholinergic markers, and quantified acetylcholine and choline concentrations using a colorimetric assay. We measured α7 nicotinic acetylcholine receptor (nAChR) dependent modulation of presynaptic function at distinct inputs in AIR- and CIE-exposed BLA coronal slices as a functional read-out of cholinergic neurotransmission. CIE/withdrawal upregulates the endogenous activity of α7 nAChRs, facilitating release at both GABAergic' local' interneuron and glutamatergic synaptic responses to stria terminalis (ST) stimulation, with no effect at GABAergic lateral paracapsular cells (LPCs). CIE caused a three-fold increase in BLA acetylcholine concentration, with no changes in α7 nAChR or cholinergic marker expression. These data illustrate that α7 nAChR-dependent changes in presynaptic function serve as a proxy for CIE-dependent alterations in synaptic acetylcholine levels. Thus, cholinergic projections appear to mediate CIE-induced alterations at GABA/glutamate inputs.
Keywords: acetylcholine; basolateral amygdala; patch-clamp electrophysiology; presynaptic; withdrawal.
Copyright © 2020 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures



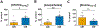
References
-
- Aitta-Aho T, Hay YA, Phillips BU, Saksida LM, Bussey TJ, Paulsen O, Apergis-Schoute J (2018). Basal forebrain and brainstem cholinergic neurons differentially impact amygdala circuits and learning-related behavior. Curr Biol, 28, 2557–2569.e4. - PubMed
-
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX (1992). Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol, 41, 802–8. - PubMed
-
- Andreasen M, Hablitz JJ (1994). Paired-pulse facilitation in the dentate gyrus: A patch-clamp study in rat hippocampus in vitro. J Neurophysiol, 72, 326–36. - PubMed
-
- Arendt T, Allen Y, Marchbanks RM, Schugens MM, Sinden J, Lantos PL, Gray JA (1989). Cholinergic system and memory in the rat: Effects of chronic ethanol, embryonic basal forebrain brain transplants and excitotoxic lesions of cholinergic basal forebrain projection system. Neuroscience, 33, 435–62. - PubMed
-
- Arvidsson U, Riedl M, Elde R, Meister B (1997). Vesicular acetylcholine transporter (vacht) protein: A novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol, 378, 454–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

