Fast computational optimization of TMS coil placement for individualized electric field targeting
- PMID: 33385544
- PMCID: PMC7956218
- DOI: 10.1016/j.neuroimage.2020.117696
Fast computational optimization of TMS coil placement for individualized electric field targeting
Abstract
Background: During transcranial magnetic stimulation (TMS) a coil placed on the scalp is used to non-invasively modulate activity of targeted brain networks via a magnetically induced electric field (E-field). Ideally, the E-field induced during TMS is concentrated on a targeted cortical region of interest (ROI). Determination of the coil position and orientation that best achieve this objective presently requires a large computational effort.
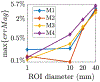
Objective: To improve the accuracy of TMS we have developed a fast computational auxiliary dipole method (ADM) for determining the optimum coil position and orientation. The optimum coil placement maximizes the E-field along a predetermined direction or, alternatively, the overall E-field magnitude in the targeted ROI. Furthermore, ADM can assess E-field uncertainty resulting from precision limitations of TMS coil placement protocols.
Method: ADM leverages the electromagnetic reciprocity principle to compute rapidly the TMS induced E-field in the ROI by using the E-field generated by a virtual constant current source residing in the ROI. The framework starts by solving for the conduction currents resulting from this ROI current source. Then, it rapidly determines the average E-field induced in the ROI for each coil position by using the conduction currents and a fast-multipole method. To further speed-up the computations, the coil is approximated using auxiliary dipoles enabling it to represent all coil orientations for a given coil position with less than 600 dipoles.
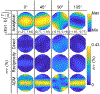
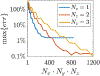
Results: Using ADM, the E-fields generated in an MRI-derived head model when the coil is placed at 5900 different scalp positions and 360 coil orientations per position (over 2.1 million unique configurations) can be determined in under 15 min on a standard laptop computer. This enables rapid extraction of the optimum coil position and orientation as well as the E-field variation resulting from coil positioning uncertainty. ADM is implemented in SimNIBS 3.2.
Conclusion: ADM enables the rapid determination of coil placement that maximizes E-field delivery to a specific brain target. This method can find the optimum coil placement in under 15 min enabling its routine use for TMS. Furthermore, it enables the fast quantification of uncertainty in the induced E-field due to limited precision of TMS coil placement protocols, enabling minimization and statistical analysis of the E-field dose variability.
Keywords: Auxiliary dipole method; Coil; E-field; Model; Optimal; Ranscranial magnetic stimulation; Reciprocity; TMS; Targeting.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest declaration A. V. Peterchev is inventor on patents and patent applications related to TMS and, in the past 3 years, has received travel funds as well as patent royalties from Rogue Research; research grants, travel funds, consulting fees, as well as equipment donation from Tal Medical / Neurex; research grant, hardware donations, and patent application support from Magstim; equipment loans and hardware donations from MagVenture; and consulting fees from Neuronetics, BTL Industries, and ACI.
Figures








References
-
- Balslev D, Braet W, McAllister C, Miall RC, 2007. Inter-individual variability in optimal current direction for transcranial magnetic stimulation of the motor cortex. J. Neurosci. Methods 162, 309–313. - PubMed
-
- Barker AT, Jalinous R, Freeston IL, 1985. Non-invasive magnetic stimulation of human motor cortex. Lancet 325, 1106–1107. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

