Landscape and Dynamics of the Transcriptional Regulatory Network During Natural Killer Cell Differentiation
- PMID: 33385611
- PMCID: PMC8377244
- DOI: 10.1016/j.gpb.2020.12.003
Landscape and Dynamics of the Transcriptional Regulatory Network During Natural Killer Cell Differentiation
Abstract
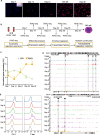
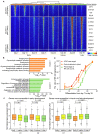
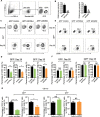
Natural killer (NK) cells are essential in controlling cancer and infection. However, little is known about the dynamics of the transcriptional regulatory machinery during NK cell differentiation. In this study, we applied the assay of transposase accessible chromatin with sequencing (ATAC-seq) technique in a home-developed in vitro NK cell differentiation system. Analysis of ATAC-seq data illustrated two distinct transcription factor (TF) clusters that dynamically regulate NK cell differentiation. Moreover, two TFs from the second cluster, FOS-like 2 (FOSL2) and early growth response 2 (EGR2), were identified as novel essential TFs that control NK cell maturation and function. Knocking down either of these two TFs significantly impacted NK cell differentiation. Finally, we constructed a genome-wide transcriptional regulatory network that provides a better understanding of the regulatory dynamics during NK cell differentiation.
Keywords: ATAC-seq; Dynamic regulatory network; EGR2; FOSL2; NK cell; Programmed differentiation.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Profiling of chromatin accessibility identifies transcription factor binding sites across the genome of Aspergillus species.BMC Biol. 2021 Sep 6;19(1):189. doi: 10.1186/s12915-021-01114-0. BMC Biol. 2021. PMID: 34488759 Free PMC article.
-
Regulatory chromatin landscape in Arabidopsis thaliana roots uncovered by coupling INTACT and ATAC-seq.Plant Methods. 2018 Dec 20;14:113. doi: 10.1186/s13007-018-0381-9. eCollection 2018. Plant Methods. 2018. PMID: 30598689 Free PMC article.
-
[Advances in assay for transposase-accessible chromatin with high-throughput sequencing].Yi Chuan. 2020 Apr 20;42(4):333-346. doi: 10.16288/j.yczz.19-279. Yi Chuan. 2020. PMID: 32312702 Review. Chinese.
-
The landscape of accessible chromatin in bovine oocytes and early embryos.Epigenetics. 2021 Mar;16(3):300-312. doi: 10.1080/15592294.2020.1795602. Epub 2020 Jul 20. Epigenetics. 2021. PMID: 32663104 Free PMC article.
-
Interrogating the Accessible Chromatin Landscape of Eukaryote Genomes Using ATAC-seq.Methods Mol Biol. 2021;2243:183-226. doi: 10.1007/978-1-0716-1103-6_10. Methods Mol Biol. 2021. PMID: 33606259 Review.
Cited by
-
Making a Killer: Selecting the Optimal Natural Killer Cells for Improved Immunotherapies.Front Immunol. 2021 Oct 27;12:765705. doi: 10.3389/fimmu.2021.765705. eCollection 2021. Front Immunol. 2021. PMID: 34777383 Free PMC article. Review.
-
Eubacterium rectale Improves the Efficacy of Anti-PD1 Immunotherapy in Melanoma via l-Serine-Mediated NK Cell Activation.Research (Wash D C). 2023 Apr 28;6:0127. doi: 10.34133/research.0127. eCollection 2023. Research (Wash D C). 2023. PMID: 37223471 Free PMC article.
-
NKG2A genetic deletion promotes human primary NK cell anti-tumor responses better than an anti-NKG2A monoclonal antibody.Mol Ther. 2024 Aug 7;32(8):2711-2727. doi: 10.1016/j.ymthe.2024.06.034. Epub 2024 Jun 27. Mol Ther. 2024. PMID: 38943249 Free PMC article.
-
PMF-GRN: a variational inference approach to single-cell gene regulatory network inference using probabilistic matrix factorization.Genome Biol. 2024 Apr 8;25(1):88. doi: 10.1186/s13059-024-03226-6. Genome Biol. 2024. PMID: 38589899 Free PMC article.
-
Natural killer cells and innate lymphoid cells 1 tune anxiety-like behavior and memory in mice via interferon-γ and acetylcholine.Nat Commun. 2023 May 29;14(1):3103. doi: 10.1038/s41467-023-38899-3. Nat Commun. 2023. PMID: 37248289 Free PMC article.
References
-
- Sun J.C. Transcriptional control of NK cells. Curr Top Microbiol Immunol. 2016;395:1–36. - PubMed
-
- Miller J.S., Soignier Y., Panoskaltsis-Mortari A., McNearney S.A., Yun G.H., Fautsch S.K. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. - PubMed
-
- Miller J.S., Verfaillie C., McGlave P. The generation of human natural killer cells from CD34+/DR− primitive progenitors in long-term bone marrow culture. Blood. 1992;80:2182–2187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

