Evidence that seasonal malaria chemoprevention with SPAQ influences blood and pre-erythrocytic stage antibody responses of Plasmodium falciparum infections in Niger
- PMID: 33386070
- PMCID: PMC7775624
- DOI: 10.1186/s12936-020-03550-9
Evidence that seasonal malaria chemoprevention with SPAQ influences blood and pre-erythrocytic stage antibody responses of Plasmodium falciparum infections in Niger
Abstract
Background: In endemic areas, children develop slowly and naturally anti-Plasmodium antibodies and become semi-immune. Seasonal Malaria Chemoprevention (SMC) with sulfadoxine-pyrimethamine + amodiaquine (SPAQ) is a new strategy to reduce malaria morbidity in West African young children. However, SMC may impact on the natural acquisition of anti-Plasmodium immunity. This paper evaluates the effect of SMC with SPAQ on antibody concentration in young children from Niger.
Methods: This research was conducted in areas benefitting from SMC since 2014 (Zinder district), without SMC (Dosso district), and with 1 year of SMC since 2016 (Gaya district). To assess the relationship between SMC and Plasmodium falciparum IgG antibody responses, the total antibody concentrations against two P. falciparum asexual stage vaccine candidate antigens, circumsporozoite protein (CSP) and glutamate-rich protein R2 (GLURP-R2), in children aged 3 to 59 months across the three areas were compared. Antibody concentrations are quantified using an enzyme-linked immunosorbent assay on the elution extracted from positive and negative malaria Rapid Diagnostic Test cassettes.
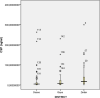
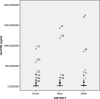
Results: The analysis concerns two hundred and twenty-nine children aged from 3 to 59 months: 71 in Zinder, 77 in Dosso, and 81 in Gaya. In Zinder (CSP = 17.5 µg/ml and GLURP-R2 = 14.3 µg/ml) median antibody concentration observed are higher than in Gaya (CSP = 7.7 µg/ml and GLURP-R2 = 6.5 µg/ml) and Dosso (CSP = 4.5 µg/ml and GLURP-R2 = 3.6 µg/ml) (p < 0.0001).
Conclusion: The research reveals some evidences which show that seasonal malaria chemoprevention with SPAQ has an effect on blood stage antibody responses and pre-erythrocytic stage of P. falciparum infections in Niger. Increased antibody titres with increased SMC/SPAQ implementation. This contradicts hypothesis that SMC/SPAQ could reduce immunity to erythrocyte and liver-stage antigens. Further studies are necessary to provide better understanding of the SMC effect on malaria immunity.
Keywords: Antibody; CSP; GLURP-R2; Immunity; P. falciparum; Seasonal malaria chemoprevention.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Asymptomatic Plasmodium falciparum infections and determinants of carriage in a seasonal malaria chemoprevention setting in Northern Cameroon and south Senegal (Kedougou).Malar J. 2024 Dec 18;23(1):386. doi: 10.1186/s12936-024-05150-3. Malar J. 2024. PMID: 39696387 Free PMC article.
-
Molecular markers of resistance to amodiaquine plus sulfadoxine-pyrimethamine in an area with seasonal malaria chemoprevention in south central Niger.Malar J. 2018 Feb 27;17(1):98. doi: 10.1186/s12936-018-2242-4. Malar J. 2018. PMID: 29486766 Free PMC article.
-
Seasonal malaria chemoprevention and the spread of Plasmodium falciparum quintuple-mutant parasites resistant to sulfadoxine-pyrimethamine: a modelling study.Lancet Microbe. 2024 Sep;5(9):100892. doi: 10.1016/S2666-5247(24)00115-0. Epub 2024 Jul 9. Lancet Microbe. 2024. PMID: 38996497
-
Systematic Review and Meta-Analysis of Seasonal Malaria Chemoprevention.Am J Trop Med Hyg. 2023 Dec 11;110(1):20-31. doi: 10.4269/ajtmh.23-0481. Print 2024 Jan 3. Am J Trop Med Hyg. 2023. PMID: 38081050 Free PMC article.
-
Antibody Correlates of Protection from Clinical Plasmodium falciparum Malaria in an Area of Low and Unstable Malaria Transmission.Am J Trop Med Hyg. 2020 Dec;103(6):2174-2182. doi: 10.4269/ajtmh.18-0805. Epub 2020 Oct 27. Am J Trop Med Hyg. 2020. PMID: 33124533 Free PMC article. Review.
Cited by
-
A roadmap for understanding sulfadoxine-pyrimethamine in malaria chemoprevention.Parasitology. 2025 Feb;152(2):133-142. doi: 10.1017/S0031182025000071. Parasitology. 2025. PMID: 39844654 Free PMC article. Review.
-
New Pyridobenothiazolone Derivatives Display Nanomolar Pan-Serotype Anti-Dengue Virus Activity.ChemMedChem. 2025 Jul 1;20(13):e202500163. doi: 10.1002/cmdc.202500163. Epub 2025 Apr 21. ChemMedChem. 2025. PMID: 40192150 Free PMC article.
-
Molecular docking and molecular dynamics simulation studies of inhibitor candidates against Anopheles gambiae 3-hydroxykynurenine transaminase and implications on vector control.Heliyon. 2025 Jan 2;11(1):e41633. doi: 10.1016/j.heliyon.2025.e41633. eCollection 2025 Jan 15. Heliyon. 2025. PMID: 39866405 Free PMC article.
-
Detection of low frequency artemisinin resistance mutations, C469Y, P553L and A675V, and fixed antifolate resistance mutations in asymptomatic primary school children in Kenya.BMC Infect Dis. 2025 Jan 16;25(1):73. doi: 10.1186/s12879-025-10462-z. BMC Infect Dis. 2025. PMID: 39819451 Free PMC article.
-
Plasmodium falciparum infection and naturally acquired immunity to malaria antigens among Ghanaian children in northern Ghana.Parasite Epidemiol Control. 2023 Jul 20;22:e00317. doi: 10.1016/j.parepi.2023.e00317. eCollection 2023 Aug. Parasite Epidemiol Control. 2023. PMID: 37501921 Free PMC article.
References
-
- OMS/WHO. Rapport sur le paludisme dans le monde 2015: résumé. Geneva, World Health Organization, 2016. http://apps.who.int/iris/handle/10665/205422. Accessed 12 Oct 2016.
-
- Systeme National d’Information Sanitaire - Niger. Données sur le paludisme http://snis.cermes.net/donnees.php. Accessed 13 Mar 2016.
-
- OMS. Recommandation de politique générale de l’OMS: Chimioprévention du paludisme saisonnier pour lutter contre le paludisme à Plasmodium falciparum en zone de forte transmission saisonnière dans la sous région du Sahel en Afrique. Geneva, World Health Organization. http://www.who.int/malaria/publications/atoz/who_smc_policy_recommendati.... Accessed 23 Nov 2014.
-
- Salissou I, Moustapha LM, Yerima B, Alkassoum I, Hadiza D, Ibrahim ML. Perception de la chimioprévention du paludisme saisonnier au Niger. Int J Biol Chem Sci. 2016;10:2710–2715. doi: 10.4314/ijbcs.v10i6.24. - DOI
-
- Salissou I, Moustapha LM, Alkassoum I, Hadiza D, Ibrahim ML. Estimation de l’impact en santé publique de la chimioprévention du paludisme saisonnier au Niger. Int J Biol Chem Sci. 2017;11:685–693. doi: 10.4314/ijbcs.v11i2.12. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

