Early Events Triggering the Initiation of a Type 2 Immune Response
- PMID: 33386241
- PMCID: PMC9813923
- DOI: 10.1016/j.it.2020.11.006
Early Events Triggering the Initiation of a Type 2 Immune Response
Abstract
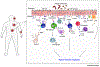
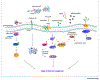
Type 2 immune responses are typically associated with protection against helminth infections and also with harmful inflammation in response to allergens. Recent advances have revealed that type 2 immunity also contributes to sterile inflammation, cancer, and microbial infections. However, the early events that initiate type 2 immune responses remain poorly defined. New insights reveal major contributions from danger-associated molecular patterns (DAMPs) in the initiation of type 2 immune responses. In this review, we examine the molecules released by the host and pathogens and the role they play in mediating the initiation of mammalian innate type 2 immune responses under a variety of conditions.
Keywords: DAMPs; allergens; cancer; helminths; sterile inflammation.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

