Diverse functions of myosin VI in spermiogenesis
- PMID: 33386429
- PMCID: PMC8021524
- DOI: 10.1007/s00418-020-01954-x
Diverse functions of myosin VI in spermiogenesis
Abstract
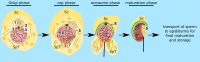
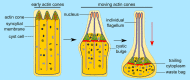
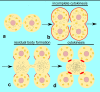
Spermiogenesis is the final stage of spermatogenesis, a differentiation process during which unpolarized spermatids undergo excessive remodeling that results in the formation of sperm. The actin cytoskeleton and associated actin-binding proteins play crucial roles during this process regulating organelle or vesicle delivery/segregation and forming unique testicular structures involved in spermatid remodeling. In addition, several myosin motor proteins including MYO6 generate force and movement during sperm differentiation. MYO6 is highly unusual as it moves towards the minus end of actin filaments in the opposite direction to other myosin motors. This specialized feature of MYO6 may explain the many proposed functions of this myosin in a wide array of cellular processes in animal cells, including endocytosis, secretion, stabilization of the Golgi complex, and regulation of actin dynamics. These diverse roles of MYO6 are mediated by a range of specialized cargo-adaptor proteins that link this myosin to distinct cellular compartments and processes. During sperm development in a number of different organisms, MYO6 carries out pivotal functions. In Drosophila, the MYO6 ortholog regulates actin reorganization during spermatid individualization and male KO flies are sterile. In C. elegans, the MYO6 ortholog mediates asymmetric segregation of cytosolic material and spermatid budding through cytokinesis, whereas in mice, this myosin regulates assembly of highly specialized actin-rich structures and formation of membrane compartments to allow the formation of fully differentiated sperm. In this review, we will present an overview and compare the diverse function of MYO6 in the specialized adaptations of spermiogenesis in flies, worms, and mammals.
Keywords: Actin; C. elegans; Drosophila; Mouse; Myosin VI; Spermiogenesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Adams A, Vogl AW. High resolution localization of Rab5, EEA1, and nectin-3 to tubulobulbar complexes in the rat testis. Anat Rec (Hoboken) 2017;300:1160–1170. - PubMed
-
- Adams A, Sriram A, Vogl AW. Internalization of intact intercellular junctions in the testis by clathrin/actin-mediated endocytic structures: tubulobulbar complexes. Anat Rec (Hoboken) 2018;301:2080–2085. - PubMed
-
- Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic. 2007;8:998–1006. - PubMed
-
- Anahara R, Toyama Y, Maekawa M, Kai M, Ishino F, Toshimori K, Mori C. Flutamide depresses expression of cortactin in the ectoplasmic specialization between the Sertoli cells and spermatids in the mouse testis. Food Chem Toxic. 2006;44:1050–1056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

