Physiological and pathological functions of TMEM106B: a gene associated with brain aging and multiple brain disorders
- PMID: 33386471
- PMCID: PMC8049516
- DOI: 10.1007/s00401-020-02246-3
Physiological and pathological functions of TMEM106B: a gene associated with brain aging and multiple brain disorders
Abstract
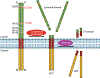
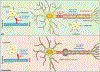
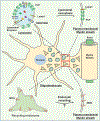
TMEM106B, encoding a lysosome membrane protein, has been recently associated with brain aging, hypomyelinating leukodystrophy and multiple neurodegenerative diseases, such as frontotemporal lobar degeneration (FTLD) and limbic-predominant age-related TDP-43 encephalopathy (LATE). During the past decade, considerable progress has been made towards our understanding of the cellular and physiological functions of TMEM106B. TMEM106B regulates many aspects of lysosomal function, including lysosomal pH, lysosome movement, and lysosome exocytosis. Both an increase and decrease in TMEM106B levels result in lysosomal abnormalities. In mouse models, TMEM106B deficiency leads to lysosome trafficking and myelination defects and FTLD related pathology. In humans, alterations in TMEM106B levels or function are intimately linked to neuronal proportions, brain aging, and brain disorders. Further elucidation of the physiological function of TMEM106B and changes in TMEM106B under pathological conditions will facilitate therapeutic development to treat brain disorders associated with TMEM106B.
Keywords: Aging; FTLD; Lysosome; Myelination; Progranulin; TDP-43; TMEM106B.
Figures
References
-
- Adams HH, Verhaaren BF, Vrooman HA, Uitterlinden AG, Hofman A, van Duijn CM, vander Lugt A, Niessen WJ, Vernooij MW, Ikram MA (2014) TMEM106B influences volume of left-sided temporal lobe and interhemispheric structures in the general population. Biol Psychiatry 76:503–508. doi: 10.1016/j.biopsych.2014.03.006 - DOI - PubMed
-
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919 - PubMed
-
- Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE, DeSaro P, Boylan KB, Graff-Radford NR, Wszolek ZK, Rademakers R, Boeve BF, McKee AC, Dickson DW (2015) Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 130:877–889. doi: 10.1007/s00401-015-1502-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

