Netrin-1 regulates ERK1/2 signaling pathway and autophagy activation in wear particle-induced osteoclastogenesis
- PMID: 33386763
- PMCID: PMC8048890
- DOI: 10.1002/cbin.11544
Netrin-1 regulates ERK1/2 signaling pathway and autophagy activation in wear particle-induced osteoclastogenesis
Abstract
Background: Artificial joint replacement surgery is often accompanied by osteolysis induced aseptic loosening around the prosthesis. Wear particles from joint replacement are thought to be one of the main factors leading to local inflammation and osteolysis at the prosthesis site. The aim of this study was to investigate the molecular mechanism of osteoclast formation and dissolution induced by wear particles and the potential roles of Netrin-1, the ERK1/2 pathway and autophagy activation in this process.
Methods: The messenger RNA levels in cells and tissues were detected with real-time quantitative PCR. The western blotting was used to detect the expression of proteins. A CCK-8 kit was used to detect the viability of RAW 264.7 cells. Moreover, an air pouch model of bone resorption was established. Immunohistochemistry was used to detect the expression of TRAP and Netrin-1 in rat bone tissue. Cell culture supernatants were collected in the rat air pouch model of bone resorption, and the levels of RANKL and OPG were detected with enzyme-linked immunosorbent assay. The protein levels of TRAP and Netrin-1 in bone tissue were examined by immunohistochemistry.
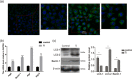
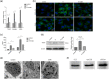
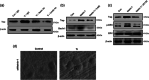
Results: Titanium wear particles induced osteoclast formation and autophagy activation. Moreover, blocking autophagy suppressed the osteoclastogenesis after exposure to wear particles in vitro. The activation of the ERK1/2 pathway and the overexpression of Netrin-1 were both found to play important roles in osteoclastogenesis mediated by autophagy. Moreover, 3-MA effectively decreased the secretion of proinflammatory cytokines mediated by wear particles.
Conclusion: Blockade of autophagy inhibits the osteoclastogenesis and inflammation induced by wear particles, thus potentially providing novel treatment strategies for abnormal osteoclastogenesis and aseptic prosthesis loosening induced by wear particles.
Keywords: ERK1/2 signaling pathway; Netrin-1; RAW 264.7 cell morphology; abnormal osteoclastogenesis; the air pouch model of bone resorption; titanium (Ti) wear particles.
© 2021 The Authors. Cell Biology International published by John Wiley & Sons Ltd on behalf of International Federation of Cell Biology.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures



References
-
- Bai, L. , Mei, X. , Shen, Z. , Bi, Y. , Yuan, Y. , Guo, Z. , Wang, H. , Zhao, H. , Zhou, Z. , Wang, C. , Zhu, K. , Li, G. , & Lv, G. (2017). Netrin‐1 improves functional recovery through autophagy regulation by activating the AMPK/mTOR signaling pathway in rats with spinal cord injury. Scientific Reports, 7, 42288. - PMC - PubMed
-
- Bouhidel, J. O. , Wang, P. , Siu, K. L. , Li, H. , Youn, J. Y. , & Cai, H. (2015). Netrin‐1 improves post‐injury cardiac function in vivo via DCC/NO‐dependent preservation of mitochondrial integrity, while attenuating autophagy. Biochimica et Biophysica Acta/General Subjects, 1852, 277–289. - PMC - PubMed
-
- Bozic, K. J. , Kurtz, S. M. , Lau, E. , Ong, K. , Vail, T. P. , & Berry, D. J. (2009). The epidemiology of revision total hip arthroplasty in the United States. The Journal of Bone and Joint Surgery. American Volume, 91, 128–133. - PubMed
-
- Brinkmann, J. 1. , Hefti, T. , Schlottig, F. , Spencer, N. D. , & Hall, H. (2012). Response of osteoclasts to titanium surfaces with increasing surface roughness: An in vitro study. Biointerphases, 7, 34. - PubMed
-
- Castillo, E. F. , Dekonenko, A. , Arko‐Mensah, J. , Mandell, M. A. , Dupont, N. , Jiang, S. , Delgado‐Vargas, M. , Timmins, G. S. , Bhattacharya, D. , Yang, H. , Hutt, J. , Lyons, C. R. , Dobos, K. M. , & Deretic, V. (2012). Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proceedings of the National Academy of Sciences of the United States of America, 109, E3168–E3176. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

