Phosphorylation and Dephosphorylation of Tau Protein by the Catalytic Subunit of PKA, as Probed by Electrophoretic Mobility Retard
- PMID: 33386804
- PMCID: PMC7990467
- DOI: 10.3233/JAD-201077
Phosphorylation and Dephosphorylation of Tau Protein by the Catalytic Subunit of PKA, as Probed by Electrophoretic Mobility Retard
Abstract
Background: Tau is a microtubule associated protein that regulates the stability of microtubules and the microtubule-dependent axonal transport. Its hyperphosphorylated form is one of the hallmarks of Alzheimer's disease and other tauopathies and the major component of the paired helical filaments that form the abnormal proteinaceous tangles found in these neurodegenerative diseases. It is generally accepted that the phosphorylation extent of tau is the result of an equilibrium in the activity of protein kinases and phosphatases. Disruption of the balance between both types of enzyme activities has been assumed to be at the origin of tau hyperphosphorylation and the subsequent toxicity and progress of the disease.
Objective: We explore the possibility that, beside the phosphatase action on phosphorylated tau, the catalytic subunit of PKA catalyzes both tau phosphorylation and also tau dephosphorylation, depending on the ATP/ADP ratio.
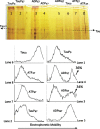
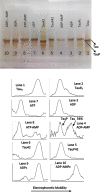
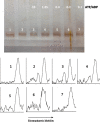
Methods: We use the shift in the relative electrophoretic mobility suffered by different phosphorylated forms of tau, as a sensor of the catalytic action of the enzyme.
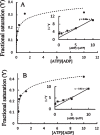
Results: The results are in agreement with the long-known thermodynamic reversibility of the phosphorylation reaction (ATP + Protein = ADP+Phospho-Protein) catalyzed by PKA and many other protein kinases.
Conclusion: The results contribute to put the compartmentalized energy state of the neuron and the mitochondrial-functions disruption upstream of tau-related pathologies.
Keywords: Dephosphorylation; PKA; electrophoretic mobility; phosphorylation; tau protein; thermodynamic reversibility.
Conflict of interest statement
Authors’ disclosures available online (
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

