SARS-CoV-2 infection in patients with primary central nervous system lymphoma
- PMID: 33387015
- PMCID: PMC7776286
- DOI: 10.1007/s00415-020-10311-w
SARS-CoV-2 infection in patients with primary central nervous system lymphoma
Abstract
Background: Cancer patients may be at higher risk for severe coronavirus infectious disease-19 (COVID-19); however, the outcome of Primary Central Nervous System Lymphoma (PCNSL) patients with SARS-CoV-2 infection has not been described yet.
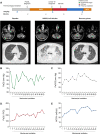
Methods: We conducted a retrospective study within the Lymphomes Oculo-Cérébraux national network (LOC) to assess the clinical characteristics and outcome of SARS-CoV-2 infection in PCNSL patients (positive real-time polymerase chain reaction of nasopharyngeal swab or evocative lung computed tomography scan). We compared clinical characteristics between patients with severe (death and/or intensive care unit admission) and mild disease.
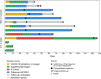
Results: Between March and May 2020, 13 PCNSL patients were diagnosed with SARS-CoV-2 infection, 11 (85%) of whom were undergoing chemotherapy at the time of infection. The mortality rate was 23% (3/13), and two additional patients (15%) required mechanical ventilation. Two patients (15%) had no COVID-19 symptoms. History of diabetes mellitus was more common in severe patients (3/5 vs 0/8, p = 0.03). Two patients recovered from COVID-19 after mechanical ventilation during more than two weeks and resumed chemotherapy. In all, chemotherapy was resumed after COVID-19 recovery in nine patients (69%) after a median delay of 16 days (range 3-32), none of whom developed unusual chemotherapy complication nor SARS-Cov2 reactivation.
Conclusion: This preliminary analysis suggests that, while being at higher risk be for severe illness, PCNSL patients with COVID-19 might be treated maximally especially if they achieved oncological response at the time of SARS-CoV-2 infection. Chemotherapy might be resumed without prolonged delay in PCNSL patients with COVID-19.
Keywords: COVID-19; PCNSL.
© 2021. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
MT reports consulting or advisory role from Agios Pharmaceutical, Integragen, and Taiho Oncology, outside the submitted work; travel, accommodations, expenses from Merck Sharp & Dome, outside the submitted work. All authors report no disclosures relevant to the manuscript.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

