Reliability and longevity of implantable defibrillators
- PMID: 33387130
- PMCID: PMC8645539
- DOI: 10.1007/s10840-020-00920-w
Reliability and longevity of implantable defibrillators
Abstract
Purpose: We hypothesized that data in manufacturers' product performance reports (PPRs) can provide clinically valuable ICD and cardiac resynchronization defibrillator (CRT-D) reliability and longevity information.
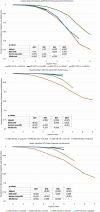
Methods: Data were obtained from 2019 PPRs. Kaplan-Meier (K-M) probabilities of freedom from malfunction, normal battery depletion (NBD), and NBD + malfunction were calculated for ICD and CRT-D pulse generators (PGs) with LiMnO2 or LiSVO/CFx batteries marketed in the USA from 2010 to 2019 and compared using the log-rank test. Malfunctions (MAL) included PGs that were found outside specifications.
Results: Study population included 1,149,803 ICD and CRT-D PGs: Abbott (ABT; 35.1%), Biotronik (BIO; 4.6%), Boston Scientific (BSC; 23.5%), and Medtronic (MDT; 36.9%). Significant differences in reliability (p < 0.001), defined by freedom from MAL, were found between manufacturers; the majority of 6808 MAL occurred in ABT devices (n = 4045; 59.4%), followed by BSC (n = 2384; 35.0%), MDT (n = 338;5.0%), and BIO (n = 41; 0.6%). Battery failure (n = 890; 57.9%) was the most common cause of MAL compromising therapy; analysis of unique ABT battery MAL-indicated problem appeared a year prior to advisory. Significant differences (p < 0.001) in battery longevity, as defined by freedom from NBD, were found between manufacturers. Overall performance (freedom from NBD + MAL) favored BSC for CRT-D PGs and MDT and BIO for ICDs. BSC subcutaneous ICD reliability was inferior to its transvenous ICD (p < 0.001).
Conclusion: PPRs contain valuable data that can be aggregated and analyzed to inform physicians. Differences in product reliability exist between manufacturers. Battery longevity has improved, but MAL have significantly impacted performance. PPR data may be useful for assessing product problems and new technology.
Keywords: Battery; Cardiac resynchronization; Implantable defibrillator; Longevity; Malfunction; Reliability.
© 2021. The Author(s).
Conflict of interest statement
Dr. Hauser: Medical Advisory Board, Cardiac Insight, Inc., Bellevue, WA, USA. The other authors declare that they have no conflict of interest.
Figures






References
-
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart malfunction. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. - DOI - PubMed
-
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, de Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart malfunction. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. - DOI - PubMed
-
- Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

