SARS-CoV-2-Induced ARDS Associates with MDSC Expansion, Lymphocyte Dysfunction, and Arginine Shortage
- PMID: 33387156
- PMCID: PMC7775842
- DOI: 10.1007/s10875-020-00920-5
SARS-CoV-2-Induced ARDS Associates with MDSC Expansion, Lymphocyte Dysfunction, and Arginine Shortage
Abstract
Purpose: The SARS-CoV-2 infection can lead to a severe acute respiratory distress syndrome (ARDS) with prolonged mechanical ventilation and high mortality rate. Interestingly, COVID-19-associated ARDS share biological and clinical features with sepsis-associated immunosuppression since lymphopenia and acquired infections associated with late mortality are frequently encountered. Mechanisms responsible for COVID-19-associated lymphopenia need to be explored since they could be responsible for delayed virus clearance and increased mortality rate among intensive care unit (ICU) patients.
Methods: A series of 26 clinically annotated COVID-19 patients were analyzed by thorough phenotypic and functional investigations at days 0, 4, and 7 after ICU admission.
Results: We revealed that, in the absence of any difference in demographic parameters nor medical history between the two groups, ARDS patients presented with an increased number of myeloid-derived suppressor cells (MDSC) and a decreased number of CD8pos effector memory cell compared to patients hospitalized for COVID-19 moderate pneumonia. Interestingly, COVID-19-related MDSC expansion was directly correlated to lymphopenia and enhanced arginase activity. Lastly, T cell proliferative capacity in vitro was significantly reduced among COVID-19 patients and could be restored through arginine supplementation.
Conclusions: The present study reports a critical role for MDSC in COVID-19-associated ARDS. Our findings open the possibility of arginine supplementation as an adjuvant therapy for these ICU patients, aiming to reduce immunosuppression and help virus clearance, thereby decreasing the duration of mechanical ventilation, nosocomial infection acquisition, and mortality.
Keywords: ARDS; Arginine; Covid-19; Cross infection; Immunosuppression; Lymphocytes; MDSC.
Conflict of interest statement
The authors declare that they have no competing interests
Figures
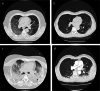
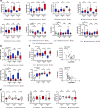
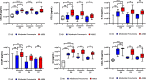
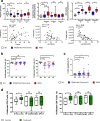
References
-
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, for the COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. - DOI - PMC - PubMed
-
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

