Evaluation of the Regulatory Review Process in Zimbabwe: Challenges and Opportunities
- PMID: 33387356
- PMCID: PMC8021537
- DOI: 10.1007/s43441-020-00242-z
Evaluation of the Regulatory Review Process in Zimbabwe: Challenges and Opportunities
Abstract
Purpose: The aims of this study were to assess the current regulatory review process of the Medicines Control Authority of Zimbabwe (MCAZ), identify key milestones and target timelines, evaluate the overall performance from 2017 to 2019, identify good review practices, evaluate the quality of decision-making processes, and identify the challenges and opportunities for improvement.
Methods: A questionnaire was completed by the MCAZ. The agency has participated in the Optimising Efficiencies in Regulatory Agencies (OpERA) program, a multinational endeavor to characterize assessment procedures and metrics associated with regulatory agencies and regional regulatory initiatives. Data identifying the milestones and overall approval times for all products registered MCAZ from 2017 to 2019 were collected and analyzed.
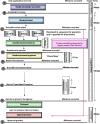
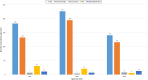
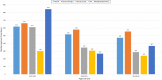
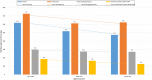
Results: The MCAZ conducts a full review of quality, safety, and efficacy data for generics and biosimilars not approved by a reference agency, an abridged review for products approved by a reference agency and a verification review for World Health Organization prequalified products under the collaborative registration procedure. The highest number of reviewed products is generics manufactured by foreign companies. There has been an improvement in review times for all categories of products over the three-year period. Guidelines, standard operating procedures, and review templates are in place and the majority of indicators for good review practices are implemented. Although quality decision-making practices are implemented, there is no formal framework in place.
Conclusion: The MCAZ successfully implements three types of review models in line with international standards. Overall, target timelines are realistic and what is achievable with the current available resources. Recommendations made such as the review of available human resources, separation of agency and company time when setting and measuring targets, review of the templates and benefit-risk framework used for abridged review, and development of a decision-making framework present opportunities for an enhanced regulatory review process.
Keywords: Good decision-making practice; Good review practices; International best practice; Medicine control authority of Zimbabwe (MCAZ); Regulatory review models; Timelines.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- World Bank. Country data: Zimbabwe 2018. https://data.worldbank.org/?locations=ZW. Accessed June 16, 2020.
-
- International Monetary Fund. Zimbabwe 2017. https://www.imf.org/en/Countries/ZWE Accessed June 16, 2020.
-
- Medicines Control Authority of Zimbabwe. http://www.mcaz.co.zw/index.php/downloads/category/7-regulations Accessed 3 April 2020.
-
- World Health Organization. Zimbabwe Medicines Quality Lab gets WHO approval. https://www.who.int/medicines/news/zwe_med_quality_lab/en/. Accessed 4 April 2020.
-
- Standards Association of Zimbabwe ISO 9001 certified companies for management systems. Pg 17 number Q320. http://saz.org.zw/registered-entities/. Accessed October 2, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

