A Fifth of the Protein World: Rossmann-like Proteins as an Evolutionarily Successful Structural unit
- PMID: 33387532
- PMCID: PMC7870570
- DOI: 10.1016/j.jmb.2020.166788
A Fifth of the Protein World: Rossmann-like Proteins as an Evolutionarily Successful Structural unit
Abstract
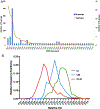
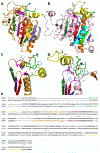
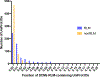
The Rossmann-like fold is the most prevalent and diversified doubly-wound superfold of ancient evolutionary origin. Rossmann-like domains are present in a variety of metabolic enzymes and are capable of binding diverse ligands. Discerning evolutionary relationships among these domains is challenging because of their diverse functions and ancient origin. We defined a minimal Rossmann-like structural motif (RLM), identified RLM-containing domains among known 3D structures (20%) and classified them according to their homologous relationships. New classifications were incorporated into our Evolutionary Classification of protein Domains (ECOD) database. We defined 156 homology groups (H-groups), which were further clustered into 123 possible homology groups (X-groups). Our analysis revealed that RLM-containing proteins constitute approximately 15% of the human proteome. We found that disease-causing mutations are more frequent within RLM domains than within non-RLM domains of these proteins, highlighting the importance of RLM-containing proteins for human health.
Keywords: Rossmann-fold; domains classification; minimal Rossmann-like motif; protein evolution.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

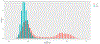

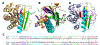
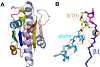
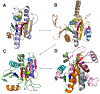
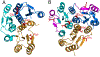


References
-
- Aravind L, Anantharaman V, & Koonin EV (2002). Monophyly of class I aminoacyl tRNA synthetase, USPA, ETFP, photolyase, and PP‐ATPase nucleotide‐binding domains: implications for protein evolution in the RNA world. Proteins. 48(1), 1–14. - PubMed
-
- Orengo CA, Jones DT, & Thornton JM (1994). Protein superfamilles and domain superfolds. Nature. 372(6507), 631–634. - PubMed
-
- Rossmann MG, Moras D, & Olsen KW (1974). Chemical and biological evolution of a nucleotide-binding protein. Nature. 250(5463), 194–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

