Biochemical Timekeeping Via Reentrant Phase Transitions
- PMID: 33387533
- PMCID: PMC8154630
- DOI: 10.1016/j.jmb.2020.166794
Biochemical Timekeeping Via Reentrant Phase Transitions
Abstract
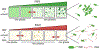
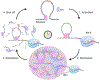
Appreciation for the role of liquid-liquid phase separation in the functional organization of cellular matter has exploded in recent years. More recently there has been a growing effort to understand the principles of heterotypic phase separation, the demixing of multiple proteins and nucleic acids into a single functional condensate. A phase transition is termed reentrant if it involves the transformation of a system from one state into a macroscopically similar or identical state via at least two phase transitions elicited by variation of a single parameter. Reentrant liquid-liquid phase separation can occur when the condensation of one species is tuned by another. Reentrant phase transitions have been modeled in vitro using protein and RNA mixtures. These biochemical studies reveal two features of reentrant phase separation that are likely important to functional cellular condensates: (1) the ability to generate condensates with layered functional topologies, and (2) the ability to generate condensates whose composition and duration are self-limiting to enable a form of biochemical timekeeping. We relate these biochemical studies to potential cellular examples and discuss how layered topologies and self-regulation may impact key biological processes.
Keywords: RNA; condensates; disordered proteins; phase separation; reentrant phase transitions; transcription.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest J.S. is a consultant for Dewpoint Therapeutics and Maze Therapeutics.
Figures
References
-
- Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, Pozniakovski A, Poser I, Maghelli N, Royer LA, Weigert M, Myers EW, Grill S, Drechsel D, Hyman AA, Alberti S, A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation, Cell 162 (2015) 1066–1077. - PubMed
-
- Mann JR, Gleixner AM, Mauna JC, Gomes E, DeChellis-Marks MR, Needham PG, Copley KE, Hurtle B, Portz B, Pyles NJ, Guo L, Calder CB, Wills ZP, Pandey UB, Kofler JK, Brodsky JL, Thathiah A, Shorter J, Donnelly CJ, RNA Binding Antagonizes Neurotoxic Phase Transitions of TDP-43, Neuron 102 (2019) 321–338.e8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

