Does sampling saliva increase detection of SARS-CoV-2 by RT-PCR? Comparing saliva with oro-nasopharyngeal swabs
- PMID: 33387561
- PMCID: PMC7833528
- DOI: 10.1016/j.jviromet.2020.114049
Does sampling saliva increase detection of SARS-CoV-2 by RT-PCR? Comparing saliva with oro-nasopharyngeal swabs
Abstract
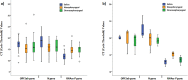
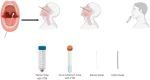
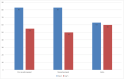
The gold standard method in the diagnosis of SARS-CoV-2 infection is the detection of viral RNA in the nasopharyngeal sample by RT-PCR. Recently, saliva samples have been suggested as an alternative sample. In the present study, we aimed to compare RT-PCR results in nasopharyngeal, oro-nasopharyngeal and saliva samples of COVID-19 patients. 98 of 200 patients were positive in RT-PCR analysis performed before the hospitalization. On day 0, at least one sample was positive in 67 % of 98 patients. The positivity rate was 83 % for both oro-nasopharyngeal and nasopharyngeal samples, while it was 63 % for saliva samples (p < 0.001). On day 5, RT-PCR was performed in 59 patients, 34 % had at least one positive result. The positivity rate was 55 % for both saliva and nasopharyngeal samples, while it was 60 % for oro-nasopharyngeal samples. Our study shows that the sampling saliva does not increase the sensitivity of RT-PCR tests at the early stages of infection. However, on the 5th day, viral RNA detection rates in saliva were similar to nasopharyngeal and oro-nasopharyngeal samples. In conclusion, we suggest that, in patients receiving treatment, RT-PCR in saliva, in addition to the standard samples, is important to determine the isolation period and control transmission.
Keywords: Coronavirus; RT-PCR; SARS-CoV-2; saliva.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures
Similar articles
-
Evaluation of sample pooling for SARS-CoV-2 RNA detection in nasopharyngeal swabs and salivas on the Roche Cobas 6800.J Clin Virol. 2021 May;138:104790. doi: 10.1016/j.jcv.2021.104790. Epub 2021 Mar 10. J Clin Virol. 2021. PMID: 33770658 Free PMC article.
-
Validation of saliva sampling as an alternative to oro-nasopharyngeal swab for detection of SARS-CoV-2 using unextracted rRT-PCR with the Allplex 2019-nCoV assay.J Med Microbiol. 2021 Aug;70(8):001404. doi: 10.1099/jmm.0.001404. J Med Microbiol. 2021. PMID: 34369860 Free PMC article.
-
Evaluation of an Automated High-Throughput Liquid-Based RNA Extraction Platform on Pooled Nasopharyngeal or Saliva Specimens for SARS-CoV-2 RT-PCR.Viruses. 2021 Apr 2;13(4):615. doi: 10.3390/v13040615. Viruses. 2021. PMID: 33918447 Free PMC article.
-
Screening for SARS-CoV-2 by RT-PCR: Saliva or nasopharyngeal swab? Rapid review and meta-analysis.PLoS One. 2021 Jun 10;16(6):e0253007. doi: 10.1371/journal.pone.0253007. eCollection 2021. PLoS One. 2021. PMID: 34111196 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
Cited by
-
Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2: A Systematic Review and Meta-analysis.JAMA Intern Med. 2021 Mar 1;181(3):353-360. doi: 10.1001/jamainternmed.2020.8876. JAMA Intern Med. 2021. PMID: 33449069 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Performance of saliva as a specimen to detect SARS-CoV-2.J Clin Virol. 2021 Sep;142:104913. doi: 10.1016/j.jcv.2021.104913. Epub 2021 Jul 17. J Clin Virol. 2021. PMID: 34304090 Free PMC article.
-
Salivary SARS-CoV-2 RNA for diagnosis of COVID-19 patients: a systematic revisew and meta-analysis of diagnostic accuracy.Jpn Dent Sci Rev. 2023 Jun 21;59:219-38. doi: 10.1016/j.jdsr.2023.06.004. Online ahead of print. Jpn Dent Sci Rev. 2023. PMID: 37360001 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous