Dexamethasone inhibits SARS-CoV-2 spike pseudotyped virus viropexis by binding to ACE2
- PMID: 33387788
- PMCID: PMC7744032
- DOI: 10.1016/j.virol.2020.12.001
Dexamethasone inhibits SARS-CoV-2 spike pseudotyped virus viropexis by binding to ACE2
Abstract
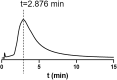
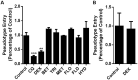
The SARS-CoV-2 outbreak, began in late 2019, has caused a worldwide pandemic and shows no signs of slowing. Glucocorticoids (GCs), including dexamethasone (DEX), have been widely used as effective anti-inflammatory and immunosuppressant drugs. In this study, seven GCs had no obvious effect on cell viability of angiotensin converting enzyme 2 (ACE2) high expressed HEK293T cells when concentrations were under 10 μM. Molecular docking results revealed that DEX occupied with active binding site of ACE2 of SARS-CoV-2 spike protein. Surface plasmon resonance (SPR) results showed that KD value between DEX and ACE2 was (9.03 ± 0.78) e-6 M. Cell membrane chromatography (CMC) results uncovered that DEX had a chromatographic retention. DEX was found out to inhibiting the viropexis into ACE2h cells using SARS-CoV-2 spike pseudotyped virus. Therefore, DEX inhibits the entrance of SARS-CoV-2 spike pseudotyped virus into cell by binding to ACE2.
Keywords: ACE2; Dexamethasone; SARS-CoV-2.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Saudi Critical Care Trial G. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

