Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19
- PMID: 33388094
- PMCID: PMC7762710
- DOI: 10.1016/j.chom.2020.12.016
Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19
Abstract
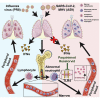
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic poses an unprecedented public health crisis. Evidence suggests that SARS-CoV-2 infection causes dysregulation of the immune system. However, the unique signature of early immune responses remains elusive. We characterized the transcriptome of rhesus macaques and mice infected with SARS-CoV-2. Alarmin S100A8 was robustly induced in SARS-CoV-2-infected animal models as well as in COVID-19 patients. Paquinimod, a specific inhibitor of S100A8/A9, could rescue the pneumonia with substantial reduction of viral loads in SARS-CoV-2-infected mice. Remarkably, Paquinimod treatment resulted in almost 100% survival in a lethal model of mouse coronavirus infection using the mouse hepatitis virus (MHV). A group of neutrophils that contributes to the uncontrolled pathological damage and onset of COVID-19 was dramatically induced by coronavirus infection. Paquinimod treatment could reduce these neutrophils and regain anti-viral responses, unveiling key roles of S100A8/A9 and aberrant neutrophils in the pathogenesis of COVID-19, highlighting new opportunities for therapeutic intervention.
Keywords: Paquinimod; S100A8/A9; SARS-CoV-2; aberrant neutrophils.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no conflicts of interest to declare.
Figures




References
-
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. - PubMed
-
- Bianchi M.E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

