White button mushroom (Agaricus bisporus) disrupts androgen receptor signaling in human prostate cancer cells and patient-derived xenograft
- PMID: 33388344
- PMCID: PMC8542389
- DOI: 10.1016/j.jnutbio.2020.108580
White button mushroom (Agaricus bisporus) disrupts androgen receptor signaling in human prostate cancer cells and patient-derived xenograft
Abstract
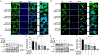
White button mushroom (WBM) (Agaricus bisporus) is a potential prostate cancer (PCa) chemo-preventative and therapeutic agent. Our clinical phase І trial of WBM powder in patients with biochemically recurrent PCa indicated that WBM intake reduced the circulating levels of prostate-specific antigen (PSA). We hypothesized that WBM exerts its effects on PCa through the androgen receptor (AR) signaling axis. Therefore, we conducted a reverse translational study with androgen-dependent PCa cell lines (LNCaP and VCaP) and patient-derived-xenografts (PDX) from a prostate tumor (TM00298). In both LNCaP and VCaP cells, western blots and qRT-PCR assays indicated that WBM extract (6-30 mg/mL) suppressed DHT-induced PSA expression and cell proliferation in a dose-dependent manner. Immunofluorescence analysis of AR revealed that WBM extract interrupted the AR nuclear-cytoplasmic distribution. PSA promotor-luciferase assay suggested that WBM extract inhibited DHT-induced luciferase activity. RNA-Seq on WBM-treated LNCaP cells confirmed that WBM treatment suppressed the androgen response pathways and cell-cycle control pathways. Our PDX showed that oral intake of WBM extract (200 mg/kg/d) suppressed tumor growth and decreased PSA levels in both tumors and serum. In the present study, we also identified a conjugated linoleic acid isomer (CLA-9Z11E) as a strong AR antagonist by performing LanthaScreen TR-FRET AR Coactivator Interaction Assays. The inhibitory effect of CLA-9Z11E (IC50: 350 nM) was nearly two times stronger than the known AR antagonist, cyproterone acetate (IC50: 672 nM). The information gained from this study improves the overall understanding of how WBM may contribute to the prevention and treatment of PCa.
Keywords: 11E)-linoleic acid; Androgen receptor; Cell cycle; Conjugated (9Z; Prostate cancer; Prostate-specific antigen; White button mushroom.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Figures



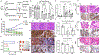
Similar articles
-
A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: Roles of cytokines and myeloid-derived suppressor cells for Agaricus bisporus-induced prostate-specific antigen responses.Cancer. 2015 Sep 1;121(17):2942-50. doi: 10.1002/cncr.29421. Epub 2015 May 18. Cancer. 2015. PMID: 25989179 Free PMC article. Clinical Trial.
-
Neoisoliquiritin exerts tumor suppressive effects on prostate cancer by repressing androgen receptor activity.Phytomedicine. 2021 May;85:153514. doi: 10.1016/j.phymed.2021.153514. Epub 2021 Feb 14. Phytomedicine. 2021. PMID: 33676083
-
Evaluation of the effects of androgenic Chinese herbal medicines on androgen receptors and tumor growth in experimental prostate cancer models.J Ethnopharmacol. 2020 Oct 5;260:113058. doi: 10.1016/j.jep.2020.113058. Epub 2020 Jun 7. J Ethnopharmacol. 2020. PMID: 32525068
-
The anticancer potential of metformin on prostate cancer.Prostate Cancer Prostatic Dis. 2019 Sep;22(3):351-361. doi: 10.1038/s41391-018-0085-2. Epub 2019 Jan 16. Prostate Cancer Prostatic Dis. 2019. PMID: 30651580 Review.
-
Recent advances in dietary androgen receptor inhibitors.Med Res Rev. 2024 Jul;44(4):1446-1500. doi: 10.1002/med.22019. Epub 2024 Jan 27. Med Res Rev. 2024. PMID: 38279967 Review.
Cited by
-
Experimental Models in Unraveling the Biological Mechanisms of Mushroom-Derived Bioactives against Aging- and Lifestyle-Related Diseases: A Review.Nutrients. 2024 Aug 13;16(16):2682. doi: 10.3390/nu16162682. Nutrients. 2024. PMID: 39203820 Free PMC article. Review.
-
Polyporus ulleungus mycelia cultured in MEB medium produce metabolites with anticancer property.J Cancer. 2024 Jan 1;15(2):309-316. doi: 10.7150/jca.89059. eCollection 2024. J Cancer. 2024. PMID: 38169554 Free PMC article.
-
Evaluation of Sample Size Influence on Chemical Characterization and In Vitro Antioxidant Properties of Flours Obtained from Mushroom Stems Coproducts.Antioxidants (Basel). 2024 Mar 14;13(3):349. doi: 10.3390/antiox13030349. Antioxidants (Basel). 2024. PMID: 38539882 Free PMC article.
-
Therapeutic Potential of Bioactive Compounds from Edible Mushrooms to Attenuate SARS-CoV-2 Infection and Some Complications of Coronavirus Disease (COVID-19).J Fungi (Basel). 2023 Aug 31;9(9):897. doi: 10.3390/jof9090897. J Fungi (Basel). 2023. PMID: 37755005 Free PMC article. Review.
-
Optimization of Mushroom (Agaricus bisporus and Pleurotus ostreatus) By-Products Processing for Prospective Functional Flour Development.Foods. 2024 Dec 14;13(24):4046. doi: 10.3390/foods13244046. Foods. 2024. PMID: 39766987 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018.68(6):394–424. - PubMed
-
- Cannata DH, Kirschenbaum A, Levine AC. Androgen deprivation therapy as primary treatment for prostate cancer. J Clin Endocrinol Meta. 2012. 97(2):360–365. - PubMed
-
- Artibani W, Porcaro AB, De Marco V, Cerruto MA, Siracusano S. Management of Biochemical Recurrence after Primary Curative Treatment for Prostate Cancer: A Review. Urol Int. 2018. 100(3):251–262. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

