Telomerase therapy attenuates cardiotoxic effects of doxorubicin
- PMID: 33388418
- PMCID: PMC8058493
- DOI: 10.1016/j.ymthe.2020.12.035
Telomerase therapy attenuates cardiotoxic effects of doxorubicin
Abstract
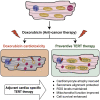
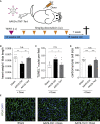
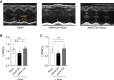
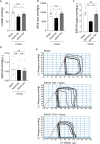
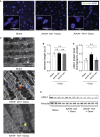
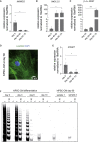
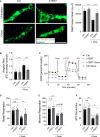
Doxorubicin is one of the most potent chemotherapeutic agents. However, its clinical use is restricted due to the severe risk of cardiotoxicity, partially attributed to elevated production of reactive oxygen species (ROS). Telomerase canonically maintains telomeres during cell division but is silenced in adult hearts. In non-dividing cells such as cardiomyocytes, telomerase confers pro-survival traits, likely owing to the detoxification of ROS. Therefore, we hypothesized that pharmacological overexpression of telomerase may be used as a therapeutic strategy for the prevention of doxorubicin-induced cardiotoxicity. We used adeno-associated virus (AAV)-mediated gene therapy for long-term expression of telomerase in in vitro and in vivo models of doxorubicin-induced cardiotoxicity. Overexpression of telomerase protected the heart from doxorubicin-mediated apoptosis and rescued cardiac function, which was accompanied by preserved cardiomyocyte size. At the mechanistic level, we observed altered mitochondrial morphology and dynamics in response to telomerase expression. Complementary in vitro experiments confirmed the anti-apoptotic effects of telomerase overexpression in human induced pluripotent stem cell-derived cardiomyocytes after doxorubicin treatment. Strikingly, elevated levels of telomerase translocated to the mitochondria upon doxorubicin treatment, which helped to maintain mitochondrial function. Thus, telomerase gene therapy could be a novel preventive strategy for cardiotoxicity by chemotherapy agents such as the anthracyclines.
Keywords: AAV gene therapy; ROS; anthracyclin; cancer; cardio-oncology; doxorubicin cardiotoxicity; heart failure; mitochondria; telomerase; telomere.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.B. and T.T. are co-founders and hold shares of Cardior Pharmaceuticals GmbH. T.T. filed and licensed patents on noncoding RNAs (outside of this paper). C. Bär. has filed and licensed patents on the therapeutic use of AAV9-mediated delivery of telomerase. The other authors declare no competing interests.
Figures

Comment in
-
Doxorubicin-induced cardiomyopathy: TERT gets to the heart of the matter.Mol Ther. 2021 Apr 7;29(4):1363-1365. doi: 10.1016/j.ymthe.2021.03.001. Epub 2021 Mar 10. Mol Ther. 2021. PMID: 33691086 Free PMC article. No abstract available.
References
-
- Curigliano G., Cardinale D., Dent S., Criscitiello C., Aseyev O., Lenihan D., Cipolla C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016;66:309–325. - PubMed
-
- Haubner B.J., Schneider J., Schweigmann U., Schuetz T., Dichtl W., Velik-Salchner C., Stein J.I., Penninger J.M. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ. Res. 2016;118:216–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

