Leukemic extracellular vesicles induce chimeric antigen receptor T cell dysfunction in chronic lymphocytic leukemia
- PMID: 33388419
- PMCID: PMC8058445
- DOI: 10.1016/j.ymthe.2020.12.033
Leukemic extracellular vesicles induce chimeric antigen receptor T cell dysfunction in chronic lymphocytic leukemia
Abstract
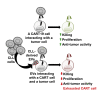
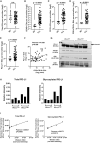
Chimeric antigen receptor (CAR) T cell therapy has yielded unprecedented outcomes in some patients with hematological malignancies; however, inhibition by the tumor microenvironment has prevented the broader success of CART cell therapy. We used chronic lymphocytic leukemia (CLL) as a model to investigate the interactions between the tumor microenvironment and CART cells. CLL is characterized by an immunosuppressive microenvironment, an abundance of systemic extracellular vesicles (EVs), and a relatively lower durable response rate to CART cell therapy. In this study, we characterized plasma EVs from untreated CLL patients and identified their leukemic cell origin. CLL-derived EVs were able to induce a state of CART cell dysfunction characterized by phenotypical, functional, and transcriptional changes of exhaustion. We demonstrate that, specifically, PD-L1+ CLL-derived EVs induce CART cell exhaustion. In conclusion, we identify an important mechanism of CART cell exhaustion induced by EVs from CLL patients.
Keywords: CART cell exhaustion; chimeric antigen receptor T cells; chronic lymphocytic leukemia; extracellular vesicles; microenvironment.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.S.K. is an inventor on patents in the field of CAR immunotherapy that are licensed to Novartis (through an agreement between the Mayo Clinic, the University of Pennsylvania, and Novartis). M.J.C., R.S., and S.S.K. are inventors on patents in the field of CAR immunotherapy that are licensed to Humanigen (through the Mayo Clinic). S.S.K. is an inventor on patents in the field of CAR immunotherapy that are licensed to Mettaforge (through the Mayo Clinic). S.S.K. receives research funding from Kite, Gilead, Juno, Celgene, Novartis, Humanigen, MorphoSys, Tolero, Sunesis, Leahlabs, and Lentigen. M.J.C., F.L., N.E.K., and S.S.K. are inventors on patents related to this work. N.E.K. receives research funding from Acerta Pharma, BMS, Pharmacyclics, MEI Pharma, and Sunesis. N.E.K. has participated in Advisory Board meetings of Cytomx Therapy, Janssen, Juno Therapeutics, AstraZeneca, and Oncotracker, and on the DSMC for Agios and Cytomx Therapeutics. S.A.P. receives research funding from Pharmacyclics, MorphoSys, Janssen, AstraZeneca, TG Therapeutics, Bristol Myers Squibb, AbbVie, and Ascentage Pharma. S.A.P. has participated in Advisory Board meetings of Pharmacyclics, AstraZeneca, Genentech, Gilead, GlaxoSmithKline, Verastem Oncology, and AbbVie (he was not personally compensated for his participation).
Figures



References
-
- Locke F.L., Ghobadi A., Jacobson C.A., Miklos D.B., Lekakis L.J., Oluwole O.O., Lin Y., Braunschweig I., Hill B.T., Timmerman J.M. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. - PMC - PubMed
-
- Siddiqi T., Soumerai J.D., Dorritie K.A., Stephens D.M., Riedell P.A., Arnason J.E., Kipps J.K., Gillenwater H.H., Gong L., Dubovsky J.A. Rapid undetectable MRD (uMRD) responses in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) treated with lisocabtagene maraleucel (liso-cel), a CD19-directed CAR T cell product: updated results from Transcend CLL 004, a phase 1/2 study including patients with high-risk disease previously treated with ibrutinib. Blood. 2019;134(Suppl 1):503.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

