Emerging Role of Ubiquitination in the Regulation of PD-1/PD-L1 in Cancer Immunotherapy
- PMID: 33388422
- PMCID: PMC7934629
- DOI: 10.1016/j.ymthe.2020.12.032
Emerging Role of Ubiquitination in the Regulation of PD-1/PD-L1 in Cancer Immunotherapy
Abstract
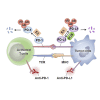
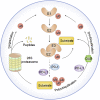
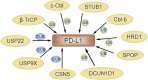
A growing amount of evidence suggests that ubiquitination and deubiquitination of programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1) play crucial roles in the regulation of PD-1 and PD-L1 protein stabilization and dynamics. PD-1/PD-L1 is a major coinhibitory checkpoint pathway that modulates immune escape in cancer patients, and its engagement and inhibition has significantly reshaped the landscape of tumor clearance. The abnormal ubiquitination and deubiquitination of PD-1/PD-L1 influence PD-1/PD-L1-mediated immunosuppression. In this review, we describe the ubiquitination- and deubiquitination-mediated modulation of PD-1/PD-L1 signaling through a variety of E3 ligases and deubiquitinating enzymes (DUBs). Moreover, we briefly expound on the anticancer potential of some agents that target related E3 ligases, which further modulate the ubiquitination of PD-1/PD-L1 in cancers. Therefore, this review reveals the development of a highly promising therapeutic approach for cancer immunotherapy by targeting PD-1/PD-L1 ubiquitination.
Keywords: PD-1; PD-L1; deubiquitination; immunotherapy; ubiquitination.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Emerging role of deubiquitination modifications of programmed death-ligand 1 in cancer immunotherapy.Front Immunol. 2023 Jun 21;14:1228200. doi: 10.3389/fimmu.2023.1228200. eCollection 2023. Front Immunol. 2023. PMID: 37415977 Free PMC article. Review.
-
Programmed Death Ligand 1 Regulatory Crosstalk with Ubiquitination and Deubiquitination: Implications in Cancer Immunotherapy.Int J Mol Sci. 2024 Mar 3;25(5):2939. doi: 10.3390/ijms25052939. Int J Mol Sci. 2024. PMID: 38474186 Free PMC article. Review.
-
The Roles of microRNAs in Regulating the Expression of PD-1/PD-L1 Immune Checkpoint.Int J Mol Sci. 2017 Nov 27;18(12):2540. doi: 10.3390/ijms18122540. Int J Mol Sci. 2017. PMID: 29186904 Free PMC article. Review.
-
Landscape of PD-1/PD-L1 Regulation and Targeted Immunotherapy.Chin Med Sci J. 2018 Sep 20;33(3):174-182. doi: 10.24920/21804. Chin Med Sci J. 2018. PMID: 30266108 Review.
-
Recent Findings in the Regulation of Programmed Death Ligand 1 Expression.Front Immunol. 2019 Jun 14;10:1337. doi: 10.3389/fimmu.2019.01337. eCollection 2019. Front Immunol. 2019. PMID: 31258527 Free PMC article. Review.
Cited by
-
At the Cutting Edge against Cancer: A Perspective on Immunoproteasome and Immune Checkpoints Modulation as a Potential Therapeutic Intervention.Cancers (Basel). 2021 Sep 28;13(19):4852. doi: 10.3390/cancers13194852. Cancers (Basel). 2021. PMID: 34638337 Free PMC article. Review.
-
PD-1 Inhibitors combined with paclitaxel (Albumin-bound) and cisplatin for larynx preservation in locally advanced laryngeal and hypopharyngeal squamous cell carcinoma: a retrospective study.Cancer Immunol Immunother. 2023 Dec;72(12):4161-4168. doi: 10.1007/s00262-023-03550-z. Epub 2023 Oct 7. Cancer Immunol Immunother. 2023. PMID: 37804437 Free PMC article.
-
Nanoparticles overcome adaptive immune resistance and enhance immunotherapy via targeting tumor microenvironment in lung cancer.Front Pharmacol. 2023 Mar 24;14:1130937. doi: 10.3389/fphar.2023.1130937. eCollection 2023. Front Pharmacol. 2023. PMID: 37033636 Free PMC article. Review.
-
Dysregulation of ubiquitination modification in renal cell carcinoma.Front Genet. 2024 Dec 19;15:1453191. doi: 10.3389/fgene.2024.1453191. eCollection 2024. Front Genet. 2024. PMID: 39748950 Free PMC article. Review.
-
The ubiquitin-proteasome system in the tumor immune microenvironment: a key force in combination therapy.Front Immunol. 2024 Sep 9;15:1436174. doi: 10.3389/fimmu.2024.1436174. eCollection 2024. Front Immunol. 2024. PMID: 39315102 Free PMC article. Review.
References
-
- Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

