Comparing COVID-19 vaccine allocation strategies in India: A mathematical modelling study
- PMID: 33388436
- PMCID: PMC7834611
- DOI: 10.1016/j.ijid.2020.12.075
Comparing COVID-19 vaccine allocation strategies in India: A mathematical modelling study
Abstract
Background: The development and widespread use of an effective SARS-CoV-2 vaccine could prevent substantial morbidity and mortality associated with COVID-19 and mitigate the secondary effects associated with non-pharmaceutical interventions.
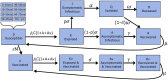
Methods: We used an age-structured, expanded SEIR model with social contact matrices to assess age-specific vaccine allocation strategies in India. We used state-specific age structures and disease transmission coefficients estimated from confirmed incident cases of COVID-19 between 1 July and 31 August 2020. Simulations were used to investigate the relative reduction in mortality and morbidity of vaccine allocation strategies based on prioritizing different age groups, and the interactions of these strategies with concurrent non-pharmaceutical interventions. Given the uncertainty associated with COVID-19 vaccine development, we varied vaccine characteristics in the modelling simulations.
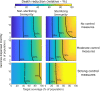
Results: Prioritizing COVID-19 vaccine allocation for older populations (i.e., >60 years) led to the greatest relative reduction in deaths, regardless of vaccine efficacy, control measures, rollout speed, or immunity dynamics. Preferential vaccination of this group often produced relatively higher total symptomatic infections and more pronounced estimates of peak incidence than other assessed strategies. Vaccine efficacy, immunity type, target coverage, and rollout speed significantly influenced overall strategy effectiveness, with the time taken to reach target coverage significantly affecting the relative mortality benefit comparative to no vaccination.
Conclusions: Our findings support global recommendations to prioritize COVID-19 vaccine allocation for older age groups. Relative differences between allocation strategies were reduced as the speed of vaccine rollout was increased. Optimal vaccine allocation strategies will depend on vaccine characteristics, strength of concurrent non-pharmaceutical interventions, and region-specific goals.
Keywords: COVID-19; Immunization; Mathematical modelling; SEIR.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Aktay A., Bavadekar S., Cossoul G., Davis J., Desfontaines D., Fabrikant A., et al. Google COVID-19 community mobility reports: anonymization process description (version 1.0) arXiv. 2020 preprint arXiv.
-
- Callaway E. 2020. COVID Vaccine Excitement Builds as Moderna Reports Third Positive Result. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

