mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach
- PMID: 33388478
- PMCID: PMC7948517
- DOI: 10.1016/j.jaip.2020.12.047
mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach
Abstract
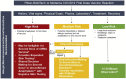
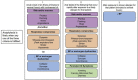
The U.S. Food and Drug Administration (FDA) has recently issued an Emergency Use Authorization (EUA) for 2 highly effective coronavirus disease 2019 (COVID-19) vaccines from Pfizer-BioNTech and Moderna. This has brought hope to millions of Americans in the midst of an ongoing global pandemic. The FDA EUA guidance for both vaccines is to not administer the vaccine to individuals with a known history of a severe allergic reaction (eg, anaphylaxis) to any component of the COVID-19 vaccine. The Centers for Disease Control and Prevention (CDC) additionally advises individuals with a history of an immediate allergic reaction to a vaccine or injectable or any history of anaphylaxis be observed for 30 minutes after COVID-19 vaccination. All other individuals should be observed for 15 minutes after COVID-19 vaccination. Staff at vaccine clinics must be able to identify and manage anaphylaxis. Post-FDA EUA, despite very strong safety signals in both phase 3 trials, reports of possible allergic reactions have raised public concern. To provide reassurance and support during widespread global vaccination, allergists must offer clear guidance to individuals based on the best information available, but also in accordance with the broader recommendations of regulatory agencies. This review summarizes vaccine allergy epidemiology and proposes drug and vaccine allergy expert opinion informed risk stratification for Allergy specialist use in conjunction with guidance of public health and regulatory authorities. The risk stratification schema guide care for (1) individuals with different allergy histories to safely receive their first mRNA COVID-19 vaccine and (2) individuals who develop a reaction to their first dose of mRNA COVID-19 vaccine.
Keywords: Allergic reactions; Allergy; Anaphylaxis; COVID-19; Guidelines; Polyethylene glycol; Polysorbate; Risk stratification; Vaccine; mRNA.
Copyright © 2020 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
The COVID-19 Pandemic in 2021: Avoiding Overdiagnosis of Anaphylaxis Risk While Safely Vaccinating the World.J Allergy Clin Immunol Pract. 2021 Apr;9(4):1438-1441. doi: 10.1016/j.jaip.2021.01.022. Epub 2021 Jan 30. J Allergy Clin Immunol Pract. 2021. PMID: 33529722 Free PMC article. No abstract available.
-
PEG skin testing for COVID-19 vaccine allergy.J Allergy Clin Immunol Pract. 2021 Apr;9(4):1765. doi: 10.1016/j.jaip.2021.02.016. J Allergy Clin Immunol Pract. 2021. PMID: 33838847 Free PMC article. No abstract available.
-
How important is the second dose of the COVID-19 mRNA vaccine?J Allergy Clin Immunol Pract. 2021 Jun;9(6):2537. doi: 10.1016/j.jaip.2021.02.061. J Allergy Clin Immunol Pract. 2021. PMID: 34112480 Free PMC article. No abstract available.
-
The hidden allergen: Triton X-100, a derivative of polyethylene glycol.J Allergy Clin Immunol Pract. 2021 Jul;9(7):2941. doi: 10.1016/j.jaip.2021.04.020. J Allergy Clin Immunol Pract. 2021. PMID: 34246443 Free PMC article. No abstract available.
-
Allergy Workup in the Diagnosis of COVID-19 Vaccines-Induced Hypersensitivity Reactions and Its Impact on Vaccination.Int Arch Allergy Immunol. 2023;184(1):54-62. doi: 10.1159/000526764. Epub 2022 Oct 20. Int Arch Allergy Immunol. 2023. PMID: 36265449 Free PMC article.
References
-
- Adeline S., Jin C.H., Hurt A., Wilburn T., Wood D., Talbot R. Coronavirus by the numbers: coronavirus is surging: how severe is your state’s outbreak? National Public Radio. December 24, 2020. https://www.npr.org/sections/health-shots/2020/09/01/816707182/map-track... Available from:
-
- Centers for Disease Control and Prevention. Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States—Appendix B. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considera... Available from:
-
- U.S. Food and Drug Administration. Moderna COVID-19 vaccine [FDA briefing document]. Silver Spring, MD: U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee; 2020. https://www.fda.gov/media/144434/download Available from:
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

