A study of the possible role of Fab-glycosylated IgG in tumor immunity
- PMID: 33388997
- PMCID: PMC10992005
- DOI: 10.1007/s00262-020-02809-z
A study of the possible role of Fab-glycosylated IgG in tumor immunity
Abstract
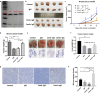
Previously we reported that administration of IgG could inhibit tumor progression in mouse models. At the same time, we also found that some IgGs have glycosylation modifications on their Fab fragments, which may have different biological functions than non-glycosylated IgG. In this study, we employed mouse tumor models to explore the roles of two different forms of IgG, i.e. Fab-glycosylated and Fab-non-glycosylated IgG, in tumor progression. The two types of IgGs were separated with ConA absorption which could react with glycan on the Fab arm but could not access glycan on the Fc fragment. In addition, we performed cytokine array, ELISA, western blotting, immunocytochemistry and other techniques to investigate the possible mechanisms of the actions of Fab-glycosylated IgG in the models. We found that Fab-glycosylated IgG, unlike Fab-non-glycosylated IgG, did not inhibit tumor growth and metastasis in the model. On the contrary, Fab-glycosylated IgG may bind to antigen-bound IgG molecules and macrophages through the glycosidic chain on the Fab fragment to affect antigen-antibody binding and macrophage polarization, which are likely to help tumor cells to evade the immune surveillance. A new mechanism of immune evasion with Fab-glycosylated IgG playing a significant role was proposed.
Keywords: Glycosylation; Immune evasion; Immunoglobulin G; Macrophage; Mouse tumor model.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

