Prostaglandin E2 Increases Neurite Length and the Formation of Axonal Loops, and Regulates Cone Turning in Differentiating NE4C Cells Via PKA
- PMID: 33389417
- PMCID: PMC11421704
- DOI: 10.1007/s10571-020-01029-4
Prostaglandin E2 Increases Neurite Length and the Formation of Axonal Loops, and Regulates Cone Turning in Differentiating NE4C Cells Via PKA
Abstract
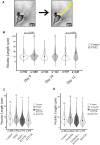
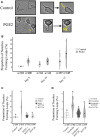
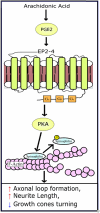
Prostaglandin E2 (PGE2) is a membrane-derived lipid signaling molecule important in neuronal development. Abnormal levels of PGE2, due to environmental insults prenatal development, have been linked to brain pathologies. We have previously shown that the addition of PGE2 to neuroectodermal (NE4C) stem cells affects early stages of neuronal differentiation (day 0-8) including increased stem cell motility, accelerated formation of neurospheres, and elevated calcium levels in growth cones. In this study, we further examine whether PGE2 can influence actin-dependent neuronal morphology in later stages (day 8-12) of NE4C cell differentiation. We show that exposure to PGE2 from the initiation of differentiation increased neurite length and the proportion of neurites that formed axonal loops. We also observed changes in the proportion of turning growth cones as the differentiation progressed, with a reduced likelihood of observing turning (or asymmetrical) growth cones on day 8 and increased odds on days 10 and 12. Moreover, we showed for the first time that the observed changes in cytoskeletal morphology were PGE2/PKA dependent. Interestingly, we also found that PGE2 decreased the total protein levels of the actin-bound form of spinophilin and increased levels of unbound PKA-phosphorylated ser94-spinophilin. Hence, we propose that exposure to PGE2 can destabilize the actin cytoskeleton at various stages of neuronal differentiation due to dissociation of ser94-spinophilin causing changes in neuronal morphology.
Keywords: Autism; Lipid signaling; Neurodevelopment; Neuronal differentiation; Prostaglandin E2; Spinophilin.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC part of Springer Nature.
Conflict of interest statement
The authors declare no conflicts of interest or competing interests.
Figures


Similar articles
-
Prostaglandin E2 promotes neural proliferation and differentiation and regulates Wnt target gene expression.J Neurosci Res. 2016 Aug;94(8):759-75. doi: 10.1002/jnr.23759. Epub 2016 Jun 5. J Neurosci Res. 2016. PMID: 27265882
-
Prostaglandin E2 elevates calcium in differentiated neuroectodermal stem cells.Mol Cell Neurosci. 2016 Jul;74:71-7. doi: 10.1016/j.mcn.2016.03.010. Epub 2016 Apr 10. Mol Cell Neurosci. 2016. PMID: 27074429
-
Prostaglandin E2 facilitates neurite outgrowth in a motor neuron-like cell line, NSC-34.J Pharmacol Sci. 2017 Oct;135(2):64-71. doi: 10.1016/j.jphs.2017.09.001. Epub 2017 Sep 8. J Pharmacol Sci. 2017. PMID: 28966102
-
Drebrin in Neuronal Migration and Axonal Growth.Adv Exp Med Biol. 2017;1006:141-155. doi: 10.1007/978-4-431-56550-5_9. Adv Exp Med Biol. 2017. PMID: 28865019 Review.
-
[Mechanisms of growth of neuronal axons and dendrites].Cesk Fysiol. 2013;62(2):47-53. Cesk Fysiol. 2013. PMID: 24392595 Review. Czech.
Cited by
-
Emodin inhibits benzidine‑enhanced survival and migration of upper urinary tract urothelial carcinoma cells by targeting the PKA/COX2 signaling pathway.Int J Oncol. 2024 Nov;65(5):103. doi: 10.3892/ijo.2024.5691. Epub 2024 Sep 20. Int J Oncol. 2024. PMID: 39301635 Free PMC article.
-
Lipid-Based Molecules on Signaling Pathways in Autism Spectrum Disorder.Int J Mol Sci. 2022 Aug 29;23(17):9803. doi: 10.3390/ijms23179803. Int J Mol Sci. 2022. PMID: 36077195 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

