Peste des petits ruminants virus non-structural C protein inhibits the induction of interferon-β by potentially interacting with MAVS and RIG-I
- PMID: 33389635
- PMCID: PMC7870622
- DOI: 10.1007/s11262-020-01811-y
Peste des petits ruminants virus non-structural C protein inhibits the induction of interferon-β by potentially interacting with MAVS and RIG-I
Abstract
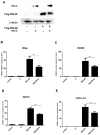
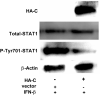
Peste des petits ruminants virus (PPRV) causes an acute and highly contagious disease in domestic and wild small ruminants throughout the world, mainly by invoking immunosuppression in its natural hosts. It has been suggested that the non-structural C protein of PPRV helps in evading host responses but the molecular mechanisms by which it antagonizes the host responses have not been fully characterized. Here, we report the antagonistic effect of PPRV C protein on the expression of interferon-β (IFN-β) through both MAVS and RIG-I mediated pathways in vitro. Dual luciferase reporter assay and direct expression of IFN-β mRNA analysis indicated that PPRV C significantly down regulates IFN-β via its potential interaction with MAVS and RIG-I signaling molecules. Results further indicated that PPRV C protein significantly suppresses endogenous and exogenous IFN-β-induced anti-viral effects in PPRV, EMCV and SVS infections in vitro. Moreover, PPRV C protein not only down regulates IFN-β but also the downstream cytokines of interferon stimulated genes 56 (ISG56), ISG15, C-X-C motif chemokine (CXCL10) and RIG-I mediated activation of IFN promoter elements of ISRE and NF-κB. Further, this study deciphers that PPRV C protein could significantly inhibit the phosphorylation of STAT1 and interferes with the signal transmission in JAK-STAT signaling pathway. Collectively, this study indicates that PPRV C protein is important for innate immune evasion and disease progression.
Keywords: Antiviral state; Interferon-β (IFN-β); Luciferase reporter assay; Pest des petits ruminants virus; Protein C.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Structure, Attachment and Transmembrane Internalisation of Peste Des Petits Ruminants Virus.Vet Med Sci. 2025 Jan;11(1):e70182. doi: 10.1002/vms3.70182. Vet Med Sci. 2025. PMID: 39739994 Free PMC article. Review.
-
Peste des Petits Ruminants Virus Nucleocapsid Protein Inhibits Beta Interferon Production by Interacting with IRF3 To Block Its Activation.J Virol. 2019 Jul 30;93(16):e00362-19. doi: 10.1128/JVI.00362-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31167907 Free PMC article.
-
The Nucleoprotein and Phosphoprotein of Peste des Petits Ruminants Virus Inhibit Interferons Signaling by Blocking the JAK-STAT Pathway.Viruses. 2019 Jul 8;11(7):629. doi: 10.3390/v11070629. Viruses. 2019. PMID: 31288481 Free PMC article.
-
Caprine MAVS Is a RIG-I Interacting Type I Interferon Inducer Downregulated by Peste des Petits Ruminants Virus Infection.Viruses. 2021 Mar 5;13(3):409. doi: 10.3390/v13030409. Viruses. 2021. PMID: 33807534 Free PMC article.
-
Role of wild small ruminants in the epidemiology of peste des petits ruminants.Transbound Emerg Dis. 2014 Oct;61(5):411-24. doi: 10.1111/tbed.12052. Epub 2013 Jan 10. Transbound Emerg Dis. 2014. PMID: 23305511 Review.
Cited by
-
Structure, Attachment and Transmembrane Internalisation of Peste Des Petits Ruminants Virus.Vet Med Sci. 2025 Jan;11(1):e70182. doi: 10.1002/vms3.70182. Vet Med Sci. 2025. PMID: 39739994 Free PMC article. Review.
-
Ribavirin inhibits peste des petits ruminants virus proliferation in vitro.Vet Med (Praha). 2023 Dec 26;68(12):464-476. doi: 10.17221/56/2023-VETMED. eCollection 2023 Dec. Vet Med (Praha). 2023. PMID: 38303996 Free PMC article.
-
Extracellular vesicles derived from PPRV-infected cells enhance signaling lymphocyte activation molecular (SLAM) receptor expression and facilitate virus infection.PLoS Pathog. 2022 Sep 9;18(9):e1010759. doi: 10.1371/journal.ppat.1010759. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36084159 Free PMC article.
-
Type I and Type II Interferon Antagonism Strategies Used by Paramyxoviridae: Previous and New Discoveries, in Comparison.Viruses. 2022 May 21;14(5):1107. doi: 10.3390/v14051107. Viruses. 2022. PMID: 35632848 Free PMC article. Review.
-
A Morbillivirus Infection Shifts DC Maturation Toward a Tolerogenic Phenotype to Suppress T Cell Activation.J Virol. 2022 Sep 28;96(18):e0124022. doi: 10.1128/jvi.01240-22. Epub 2022 Sep 12. J Virol. 2022. PMID: 36094317 Free PMC article.
References
-
- Aguilar XF, Fine AE, Pruvot M, Njeumi F, Walzer C, Kock R, Shiilegdamba E. PPR virus threatens wildlife conservation. Science. 2018;362:165–166. - PubMed
-
- Woo PCY, Lau SKP, Wong BHL, Fan RYY, Wong AYP, Zhang AJX, Wu Y, Choi GKY, Li KSM, Hui J, Wang M, Zheng BJ, Chan KH, Yuen KY. Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulointerstitial nephritis in domestic cats. Proc Natl Acad Sci USA. 2012;109:5435–5440. - PMC - PubMed
-
- Cattaneo R, Kaelin K, Baczko K, Billeter MA. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989;56(5):759–764. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

