Identification of RipAZ1 as an avirulence determinant of Ralstonia solanacearum in Solanum americanum
- PMID: 33389783
- PMCID: PMC7865085
- DOI: 10.1111/mpp.13030
Identification of RipAZ1 as an avirulence determinant of Ralstonia solanacearum in Solanum americanum
Abstract
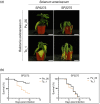
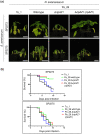
Ralstonia solanacearum causes bacterial wilt disease in many plant species. Type III-secreted effectors (T3Es) play crucial roles in bacterial pathogenesis. However, some T3Es are recognized by corresponding disease resistance proteins and activate plant immunity. In this study, we identified the R. solanacearum T3E protein RipAZ1 (Ralstonia injected protein AZ1) as an avirulence determinant in the black nightshade species Solanum americanum. Based on the S. americanum accession-specific avirulence phenotype of R. solanacearum strain Pe_26, 12 candidate avirulence T3Es were selected for further analysis. Among these candidates, only RipAZ1 induced a cell death response when transiently expressed in a bacterial wilt-resistant S. americanum accession. Furthermore, loss of ripAZ1 in the avirulent R. solanacearum strain Pe_26 resulted in acquired virulence. Our analysis of the natural sequence and functional variation of RipAZ1 demonstrated that the naturally occurring C-terminal truncation results in loss of RipAZ1-triggered cell death. We also show that the 213 amino acid central region of RipAZ1 is sufficient to induce cell death in S. americanum. Finally, we show that RipAZ1 may activate defence in host cell cytoplasm. Taken together, our data indicate that the nucleocytoplasmic T3E RipAZ1 confers R. solanacearum avirulence in S. americanum. Few avirulence genes are known in vascular bacterial phytopathogens and ripAZ1 is the first one in R. solanacearum that is recognized in black nightshades. This work thus opens the way for the identification of disease resistance genes responsible for the specific recognition of RipAZ1, which can be a source of resistance against the devastating bacterial wilt disease.
Keywords: Ralstonia solanacearum; Solanum americanum; avirulence; bacterial wilt; effector; effector-triggered immunity.
© 2021 The Authors. Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Figures




References
-
- Baggs, E. , Dagdas, G. & Krasileva, K.V. (2017) NLR diversity, helpers and integrated domains: making sense of the NLR IDentity. Current Opinion in Plant Biology, 38, 59–67. - PubMed
-
- Belbahri, L. , Boucher, C. , Candresse, T. , Nicole, M. , Ricci, P. & Keller, H. (2001) A local accumulation of the Ralstonia solancearum PopA protein in transgenic tobacco renders a compatible plant–pathogen interaction incompatible. The Plant Journal, 28, 419–430. - PubMed
-
- Bergelson, J. , Kreitman, M. , Stahl, E.A. & Tian, D. (2001) Evolutionary dynamics of plant R‐genes. Science, 292, 2281–2285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

