Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8+ T cell exclusion
- PMID: 33390170
- PMCID: PMC7780622
- DOI: 10.1186/s13046-020-01786-6
Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8+ T cell exclusion
Erratum in
-
Correction to: Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8+ T cell exclusion.J Exp Clin Cancer Res. 2022 Mar 12;41(1):93. doi: 10.1186/s13046-022-02292-7. J Exp Clin Cancer Res. 2022. PMID: 35279221 Free PMC article. No abstract available.
Abstract
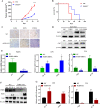
Background: Progranulin (PGRN), as a multifunctional growth factor, is overexpressed in multiple tumors, but the role of PGRN on tumor immunity is still unclear. Here, we studied the effect of PGRN on breast cancer tumor immunity and its possible molecular mechanism.
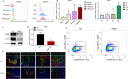
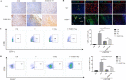
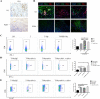
Methods: The changes of macrophage phenotypes after PGRN treatment were detected by western blot, quantitative polymerase chain reaction (PCR) and flow cytometry. Western blot was used to study the signal molecular mechanism of PGRN regulating this process. The number and localization of immune cells in Wild-type (WT) and PGRN-/- breast cancer tissues were analyzed by immunohistochemical staining and immunofluorescence techniques. The activation and proliferation of CD8+ T cells were measured by flow cytometry.
Results: After being treated with PGRN, the expressions of M2 markers and programmed death ligand 1 (PD-L1) on macrophages increased significantly. Signal transducer and activator of transcription 3 (STAT3) signaling pathway inhibitor Stattic significantly inhibited the expression of PD-L1 and M2 related markers induced by PGRN. In WT group, CD8 were co-localized with macrophages and PD-L1, but not tumor cells. The number of immune cells in PGRN-/- breast cancer tissue increased, and their infiltration into tumor parenchyma was also enhanced. Moreover, in the co-culture system, WT peritoneal macrophages not only reduced the ratio of activated CD8+ T cells but also reduced the proportion of proliferating CD8+ T cells. The addition of programmed death receptor 1 (PD-1) and PD-L1 neutralizing antibodies effectively reversed this effect and restored the immune function of CD8+ T cells.
Conclusion: These results demonstrate that PGRN promotes M2 polarization and PD-L1 expression by activating the STAT3 signaling pathway. Furthermore, through PD-1/PD-L1 interaction, PGRN can promote the breast tumor immune escape. Our research may provide new ideas and targets for clinical breast cancer immunotherapy.
Keywords: Breast cancer; PD-L1; Progranulin (PGRN); T cell; TAMs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

