Current advances in the development of SARS-CoV-2 vaccines
- PMID: 33390829
- PMCID: PMC7757035
- DOI: 10.7150/ijbs.52569
Current advances in the development of SARS-CoV-2 vaccines
Abstract
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is now a global pandemic that has wreaked havoc globally, which has put a heavy toll on public health, lives, and the world economy. Vaccination is considered as one of the greatest successes in medical history. Based on prior experience with the development of SARS-CoV vaccines, all COVID-19 vaccines must be subjected to the tests for protective effects and harmful risks derived from antibody-dependent enhancement that may contribute to augmented infectivity and/or eosinophilic infiltration. The SARS-CoV-2 vaccine is now being developed urgently in several different ways. China is regarded as one of the world's leading countries in SARS-CoV-2 vaccine development, up to date the last inactivated vaccine international clinical (Phase III) trial was launched in the United Arab Emirates by Sinopharm China National Biotec Group (CNBG). In this review, we outline the current status of vaccine development against clinically relevant SARS-CoV-2 strains, anticipating that such attempts would help create efficacious and sage SARS-CoV-2 vaccines.
Keywords: COVID-19; SARS-CoV-2; clinical trial; coronavirus; vaccines.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
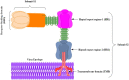
Figures


References
-
- Awadasseid A, Wu Y, Tanaka Y, Zhang W. Initial success in the identification and management of the coronavirus disease 2019 (COVID-19) indicates human-to-human transmission in Wuhan, China. International Journal of Biological Sciences. 2020;16:1846–60. http://www.ijbs.com/v16p.htm. - PMC - PubMed
-
- Control ECfDPa. European Centre for Disease Prevention and Control 2020; https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases.
-
- NHS. SARS (severe acute respiratory syndrome) https://www.nhs.uk/conditions/sars/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

