Dysfunctional Autophagy and Endolysosomal System in Neurodegenerative Diseases: Relevance and Therapeutic Options
- PMID: 33390907
- PMCID: PMC7773602
- DOI: 10.3389/fncel.2020.602116
Dysfunctional Autophagy and Endolysosomal System in Neurodegenerative Diseases: Relevance and Therapeutic Options
Abstract
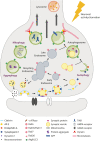
Autophagy and endolysosomal trafficking are crucial in neuronal development, function and survival. These processes ensure efficient removal of misfolded aggregation-prone proteins and damaged organelles, such as dysfunctional mitochondria, thus allowing the maintenance of proper cellular homeostasis. Beside this, emerging evidence has pointed to their involvement in the regulation of the synaptic proteome needed to guarantee an efficient neurotransmitter release and synaptic plasticity. Along this line, an intimate interplay between the molecular machinery regulating synaptic vesicle endocytosis and synaptic autophagy is emerging, suggesting that synaptic quality control mechanisms need to be tightly coupled to neurosecretion to secure release accuracy. Defects in autophagy and endolysosomal pathway have been associated with neuronal dysfunction and extensively reported in Alzheimer's, Parkinson's, Huntington's and amyotrophic lateral sclerosis among other neurodegenerative diseases, with common features and emerging genetic bases. In this review, we focus on the multiple roles of autophagy and endolysosomal system in neuronal homeostasis and highlight how their defects probably contribute to synaptic default and neurodegeneration in the above-mentioned diseases, discussing the most recent options explored for therapeutic interventions.
Keywords: autophagy; endocytosis; lysosomes; neurodegenerative diseases; synapse.
Copyright © 2020 Giovedì, Ravanelli, Parisi, Bettegazzi and Guarnieri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer FC declared a past co-authorship with one of the authors SG to the handling editor.
Figures
References
-
- Alegre-Abarrategui J., Christian H., Lufino M. M. P., Mutihac R., Venda L. L., Ansorge O., et al. (2009). LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum. Mol. Genet. 18 4022–4034. 10.1093/hmg/ddp346 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

