Postoperative Ileus and Postoperative Gastrointestinal Tract Dysfunction: Pathogenic Mechanisms and Novel Treatment Strategies Beyond Colorectal Enhanced Recovery After Surgery Protocols
- PMID: 33390950
- PMCID: PMC7774512
- DOI: 10.3389/fphar.2020.583422
Postoperative Ileus and Postoperative Gastrointestinal Tract Dysfunction: Pathogenic Mechanisms and Novel Treatment Strategies Beyond Colorectal Enhanced Recovery After Surgery Protocols
Abstract
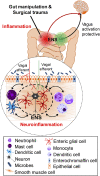
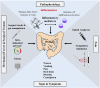
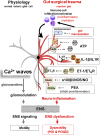
Postoperative ileus (POI) and postoperative gastrointestinal tract dysfunction (POGD) are well-known complications affecting patients undergoing intestinal surgery. GI symptoms include nausea, vomiting, pain, abdominal distention, bloating, and constipation. These iatrogenic disorders are associated with extended hospitalizations, increased morbidity, and health care costs into the billions and current therapeutic strategies are limited. This is a narrative review focused on recent concepts in the pathogenesis of POI and POGD, pipeline drugs or approaches to treatment. Mechanisms, cellular targets and pathways implicated in the pathogenesis include gut surgical manipulation and surgical trauma, neuroinflammation, reactive enteric glia, macrophages, mast cells, monocytes, neutrophils and ICC's. The precise interactions between immune, inflammatory, neural and glial cells are not well understood. Reactive enteric glial cells are an emerging therapeutic target that is under intense investigation for enteric neuropathies, GI dysmotility and POI. Our review emphasizes current therapeutic strategies, starting with the implementation of colorectal enhanced recovery after surgery protocols to protect against POI and POGD. However, despite colorectal enhanced recovery after surgery, it remains a significant medical problem and burden on the healthcare system. Over 100 pipeline drugs or treatments are listed in Clin.Trials.gov. These include 5HT4R agonists (Prucalopride and TAK 954), vagus nerve stimulation of the ENS-macrophage nAChR cholinergic pathway, acupuncture, herbal medications, peripheral acting opioid antagonists (Alvimopen, Methlnaltexone, Naldemedine), anti-bloating/flatulence drugs (Simethiocone), a ghreline prokinetic agonist (Ulimovelin), drinking coffee, and nicotine chewing gum. A better understanding of the pathogenic mechanisms for short and long-term outcomes is necessary before we can develop better prophylactic and treatment strategies.
Keywords: 5HT4 receptor; colorectal enhanced recovery after surgery; enteric glia; gastrointestinal surgery; mechanosensation; postoperative gastrointestinal tract dysfunction; postoperative ileus; prokinetic agents.
Copyright © 2020 Mazzotta, Villalobos-Hernandez, FIORDA-DIAZ, Harzman and Christofi.
Conflict of interest statement
FC, EM, and JFD are Co-Investigators on a multi-center clinical trial on postoperative ileus with TAKEDA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Adiamah A., Lobo D. N. (2020). “Postoperative ileus: prevention and treatment,” in Enhanced recovery after surgery. New York, NY: Springer International Publishing, 249–257.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

