Hydrogen Sulfide Attenuates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease by Inhibiting Apoptosis and Promoting Autophagy via Reactive Oxygen Species/Phosphatidylinositol 3-Kinase/AKT/Mammalian Target of Rapamycin Signaling Pathway
- PMID: 33390956
- PMCID: PMC7774297
- DOI: 10.3389/fphar.2020.585860
Hydrogen Sulfide Attenuates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease by Inhibiting Apoptosis and Promoting Autophagy via Reactive Oxygen Species/Phosphatidylinositol 3-Kinase/AKT/Mammalian Target of Rapamycin Signaling Pathway
Abstract
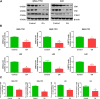
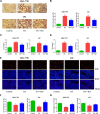
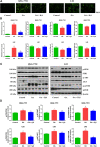
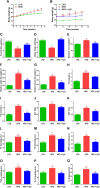
Non-alcoholic fatty liver disease (NAFLD) is a common chronic liver disease worldwide. Hydrogen sulfide (H2S) is involved in a wide range of physiological and pathological processes. Nevertheless, the mechanism of action of H2S in NAFLD development has not been fully clarified. Here, the reduced level of H2S was observed in liver cells treated with oleic acid (OA). Administration of H2S increased the proliferation of OA-treated cells. The results showed that H2S decreased apoptosis and promoted autophagy through reactive oxygen species (ROS)-mediated phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) cascade in OA-treated cells. In addition, administration of H2S relieved high-fat diet (HFD)-induced NAFLD via inhibition of apoptosis and promotion of autophagy. These findings suggest that H2S could ameliorate HFD-induced NAFLD by regulating apoptosis and autophagy through ROS/PI3K/AKT/mTOR signaling pathway. Novel H2S-releasing donors may have therapeutic potential for the treatment of NAFLD.
Keywords: apoptosis; autophagy; hydrogen sulfide; nonalcoholic fatty liver disease; signaling pathway.
Copyright © 2020 Wu, Zhong, Wang, Zhang, Li, Liu, Ji and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

