The Mysteries of Capsaicin-Sensitive Afferents
- PMID: 33391007
- PMCID: PMC7772409
- DOI: 10.3389/fphys.2020.554195
The Mysteries of Capsaicin-Sensitive Afferents
Abstract
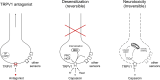
A fundamental subdivision of nociceptive sensory neurons is named after their unique sensitivity to capsaicin, the pungent ingredient in hot chili peppers: these are the capsaicin-sensitive afferents. The initial excitation by capsaicin of these neurons manifested as burning pain sensation is followed by a lasting refractory state, traditionally referred to as "capsaicin desensitization," during which the previously excited neurons are unresponsive not only to capsaicin but a variety of unrelated stimuli including noxious heat. The long sought-after capsaicin receptor, now known as TRPV1 (transient receptor potential cation channel, subfamily V member 1), was cloned more than two decades ago. The substantial reduction of the inflammatory phenotype of Trpv1 knockout mice has spurred extensive efforts in the pharmaceutical industry to develop small molecule TRPV1 antagonists. However, adverse effects, most importantly hyperthermia and burn injuries, have so far prevented any compounds from progressing beyond Phase 2. There is increasing evidence that these limitations can be at least partially overcome by approaches outside of the mainstream pharmaceutical development, providing novel therapeutic options through TRPV1. Although ablation of the whole TRPV1-expressing nerve population by high dose capsaicin, or more selectively by intersectional genetics, has allowed researchers to investigate the functions of capsaicin-sensitive afferents in health and disease, several "mysteries" remain unsolved to date, including the molecular underpinnings of "capsaicin desensitization," and the exact role these nerves play in thermoregulation and heat sensation. This review tries to shed some light on these capsaicin mechanisms.
Keywords: TRPV1 receptor; capsaicin; inflammation; ion channel; pain; sensory neuron; thermoregulation.
Copyright © 2020 Fischer, Ciotu and Szallasi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Arendt-Nielsen L., Harris S., Whiteside G. T., Hummel M., Knappenberger T., O’Keefe S., et al. (2016). A randomized, double-blind, positive-controlled, 3-way cross-over human experimental pain study of a TRPV1 antagonist (V116517) in healthy volunteers and comparison with preclinical profile. Pain 157 2057–2067. 10.1097/j.pain.0000000000000610 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

