Responses of Neurons in the Medullary Lateral Tegmental Field and Nucleus Tractus Solitarius to Vestibular Stimuli in Conscious Felines
- PMID: 33391176
- PMCID: PMC7775595
- DOI: 10.3389/fneur.2020.620817
Responses of Neurons in the Medullary Lateral Tegmental Field and Nucleus Tractus Solitarius to Vestibular Stimuli in Conscious Felines
Abstract
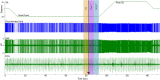
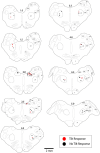
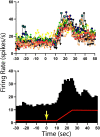
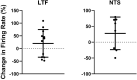
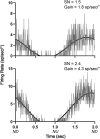
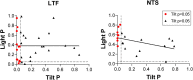
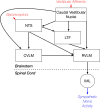
Considerable evidence shows that the vestibular system contributes to adjusting sympathetic nervous system activity to maintain adequate blood pressure during movement and changes in posture. However, only a few prior experiments entailed recordings in conscious animals from brainstem neurons presumed to convey baroreceptor and vestibular inputs to neurons in the rostral ventrolateral medulla (RVLM) that provide inputs to sympathetic preganglionic neurons in the spinal cord. In this study, recordings were made in conscious felines from neurons in the medullary lateral tegmental field (LTF) and nucleus tractus solitarius (NTS) identified as regulating sympathetic nervous system activity by exhibiting changes in firing rate related to the cardiac cycle, or cardiac-related activity (CRA). Approximately 38% of LTF and NTS neurons responded to static 40° head up tilts with a change in firing rate (increase for 60% of the neurons, decrease for 40%) of ~50%. However, few of these neurons responded to 10° sinusoidal rotations in the pitch plane, in contrast to prior findings in decerebrate animals that the firing rates of both NTS and LTF neurons are modulated by small-amplitude body rotations. Thus, as previously demonstrated for RVLM neurons, in conscious animals NTS and LTF neurons only respond to large rotations that lead to changes in sympathetic nervous system activity. The similar responses to head-up rotations of LTF and NTS neurons with those documented for RVLM neurons suggest that LTF and NTS neurons are components of the vestibulo-sympathetic reflex pathway. However, a difference between NTS/LTF and RVLM neurons was variability in CRA over time. This variability was significantly greater for RVLM neurons, raising the hypothesis that the responsiveness of these neurons to baroreceptor input is adjusted based on the animal's vigilance and alertness.
Keywords: cardiovascular regulation; lateral tegmental field; nucleus tractus solitarius (NTS); reticular formation; rostral ventrolateral medulla; sympathetic nervous system; vestibular; vestibulo-sympathetic responses.
Copyright © 2020 Bielanin, Douglas, Shulgach, McCall, Miller, Amin, Murphey, Barman and Yates.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Guyenet PG, Koshiya N, Huangfu D, Baraban SC, Stornetta RL, Li YW. Role of medulla oblongata in generation of sympathetic and vagal outflows. In: Holstege Bandler GR, Saper CB. editors. Emotional Motor System. Amsterdam: Elsevier; (1996). p. 127–44. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

