Peptidoglycan Endopeptidase Spr of Uropathogenic Escherichia coli Contributes to Kidney Infections and Competitive Fitness During Bladder Colonization
- PMID: 33391204
- PMCID: PMC7774453
- DOI: 10.3389/fmicb.2020.586214
Peptidoglycan Endopeptidase Spr of Uropathogenic Escherichia coli Contributes to Kidney Infections and Competitive Fitness During Bladder Colonization
Abstract
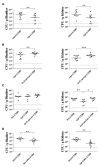
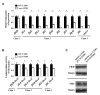
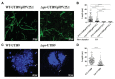
Uropathogenic E scherichia coli (UPEC) is the most common pathogen of urinary tract infections (UTIs). Antibiotic therapy is the conventional measure to manage such infections. However, the rapid emergence of antibiotic resistance has reduced the efficacy of antibiotic treatment. Given that the bacterial factors required for the full virulence of the pathogens are potential therapeutic targets, identifying such factors may facilitate the development of novel therapeutic strategies against UPEC UTIs. The peptidoglycan (PG) endopeptidase Spr (also named MepS) is required for PG biogenesis in E. coli. In the present study, we found that Spr deficiency attenuated the ability of UPEC to infect kidneys and induced a fitness defect during bladder colonization in a mouse model of UTI. Based on the liquid chromatography (LC)/mass spectrometry (MS)/MS analysis of the bacterial envelope, spr deletion changed the levels of some envelope-associated proteins, suggesting that Spr deficiency interfere with the components of the bacterial structure. Among the proteins, FliC was significantly downregulated in the spr mutant, which is resulted in reduced motility. Lack of Spr might hinder the function of the flagellar transcriptional factor FlhDC to decrease FliC expression. The motility downregulation contributed to the reduced fitness in urinary tract colonization. Additionally, spr deletion compromised the ability of UPEC to evade complement-mediated attack and to resist intracellular killing of phagocytes, consequently decreasing UPEC bloodstream survival. Spr deficiency also interfered with the UPEC morphological switch from bacillary to filamentous shapes during UTI. It is known that bacterial filamentation protects UPEC from phagocytosis by phagocytes. In conclusion, Spr deficiency was shown to compromise multiple virulence properties of UPEC, leading to attenuation of the pathogen in urinary tract colonization and bloodstream survival. These findings indicate that Spr is a potential antimicrobial target for further studies attempting to develop novel strategies in managing UPEC UTIs.
Keywords: MepS; Spr; bacteremia; flagella; motility; the complement system; urinary tract infections.
Copyright © 2020 Huang, Hashimoto, Shih, Wu, Lee, Chen, Wu, Wang, Lin, Hong and Teng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Andersen T. E., Khandige S., Madelung M., Brewer J., Kolmos H. J., Moller-Jensen J. (2012). Escherichia coli uropathogenesis in vitro: invasion, cellular escape, and secondary infection analyzed in a human bladder cell infection model. Infect. Immun. 80, 1858–1867. 10.1128/IAI.06075-11, PMID: - DOI - PMC - PubMed
-
- Barnich N., Boudeau J., Claret L., Darfeuille-Michaud A. (2003). Regulatory and functional co-operation of flagella and type 1 pili in adhesive and invasive abilities of AIEC strain LF82 isolated from a patient with Crohn’s disease. Mol. Microbiol. 48, 781–794. 10.1046/j.1365-2958.2003.03468.x, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

