Functional Prediction and Assignment of Methanobrevibacter ruminantium M1 Operome Using a Combined Bioinformatics Approach
- PMID: 33391347
- PMCID: PMC7772410
- DOI: 10.3389/fgene.2020.593990
Functional Prediction and Assignment of Methanobrevibacter ruminantium M1 Operome Using a Combined Bioinformatics Approach
Abstract
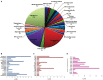
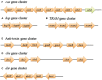
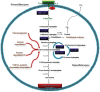
Methanobrevibacter ruminantium M1 (MRU) is a rod-shaped rumen methanogen with the ability to use H2 and CO2, and formate as substrates for methane formation in the ruminants. Enteric methane emitted from this organism can also be influential to the loss of dietary energy in ruminants and humans. To date, there is no successful technology to reduce methane due to a lack of knowledge on its molecular machinery and 73% conserved hypothetical proteins (HPs; operome) whose functions are still not ascertained perceptively. To address this issue, we have predicted and assigned a precise function to HPs and categorize them as metabolic enzymes, binding proteins, and transport proteins using a combined bioinformatics approach. The results of our study show that 257 (34%) HPs have well-defined functions and contributed essential roles in its growth physiology and host adaptation. The genome-neighborhood analysis identified 6 operon-like clusters such as hsp, TRAM, dsr, cbs and cas, which are responsible for protein folding, sudden heat-shock, host defense, and protection against the toxicities in the rumen. The functions predicted from MRU operome comprised of 96 metabolic enzymes with 17 metabolic subsystems, 31 transcriptional regulators, 23 transport, and 11 binding proteins. Functional annotation of its operome is thus more imperative to unravel the molecular and cellular machinery at the systems-level. The functional assignment of its operome would advance strategies to develop new anti-methanogenic targets to mitigate methane production. Hence, our approach provides new insight into the understanding of its growth physiology and lifestyle in the ruminants and also to reduce anthropogenic greenhouse gas emissions worldwide.
Keywords: hypothetical proteins; methane mitigation; methanobrevibacter; molecular machinery; protein function.
Copyright © 2020 Bharathi, Senthil Kumar and Chellapandi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Agnihotri G., Liu H. W. (2003). Enoyl-CoA hydratase. reaction, mechanism, and inhibition. Bioorg. Med. Chem. 11 9–20. - PubMed
LinkOut - more resources
Full Text Sources

