A role for intestinal alkaline phosphatase in preventing liver fibrosis
- PMID: 33391458
- PMCID: PMC7681079
- DOI: 10.7150/thno.48468
A role for intestinal alkaline phosphatase in preventing liver fibrosis
Abstract
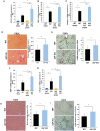
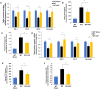
Rationale: Liver fibrosis is frequently associated with gut barrier dysfunction, and the lipopolysaccharides (LPS) -TLR4 pathway is common to the development of both. Intestinal alkaline phosphatase (IAP) has the ability to detoxify LPS, as well as maintain intestinal tight junction proteins and gut barrier integrity. Therefore, we hypothesized that IAP may function as a novel therapy to prevent liver fibrosis. Methods: Stool IAP activity from cirrhotic patients were determined. Common bile duct ligation (CBDL) and Carbon Tetrachloride-4 (CCl4)-induced liver fibrosis models were used in WT, IAP knockout (KO), and TLR4 KO mice supplemented with or without exogenous IAP in their drinking water. The gut barrier function and liver fibrosis markers were tested. Results: Human stool IAP activity was decreased in the setting of liver cirrhosis. In mice, IAP activity and genes expression decreased after CBDL and CCl4 exposure. Intestinal tight junction related genes and gut barrier function were impaired in both models of liver fibrosis. Oral IAP supplementation attenuated the decrease in small intestine tight junction protein gene expression and gut barrier function. Liver fibrosis markers were significantly higher in IAP KO compared to WT mice in both models, while oral IAP rescued liver fibrosis in both WT and IAP KO mice. In contrast, IAP supplementation did not attenuate fibrosis in TLR4 KO mice in either model. Conclusions: Endogenous IAP is decreased during liver fibrosis, perhaps contributing to the gut barrier dysfunction and worsening fibrosis. Oral IAP protects the gut barrier and further prevents the development of liver fibrosis via a TLR4-mediated mechanism.
Keywords: Liver fibrosis; TLR4; gut barrier; intestinal alkaline phosphatase.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Wang Y, Tong J, Chang B, Wang B, Zhang D, Wang B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol Med Rep. 2014;9:2352–6. - PubMed
-
- Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. - PubMed
-
- Seki E, De Minicis S, Österreicher CH, Kluwe J, Osawa Y, Brenner DA. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

