Implantation of regenerative complexes in traumatic brain injury canine models enhances the reconstruction of neural networks and motor function recovery
- PMID: 33391504
- PMCID: PMC7738861
- DOI: 10.7150/thno.50540
Implantation of regenerative complexes in traumatic brain injury canine models enhances the reconstruction of neural networks and motor function recovery
Abstract
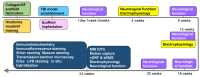
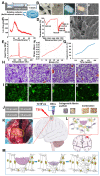
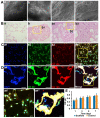
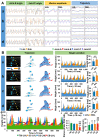
Rationale: The combination of medical and tissue engineering in neural regeneration studies is a promising field. Collagen, silk fibroin and seed cells are suitable options and have been widely used in the repair of spinal cord injury. In this study, we aimed to determine whether the implantation of a complex fabricated with collagen/silk fibroin (SF) and the human umbilical cord mesenchymal stem cells (hUCMSCs) can promote cerebral cortex repair and motor functional recovery in a canine model of traumatic brain injury (TBI). Methods: A porous scaffold was fabricated with cross-linked collagen and SF. Its physical properties and degeneration rate were measured. The scaffolds were co-cultured with hUCMSCs after which an implantable complex was formed. After complex implantation to a canine model of TBI, the motor evoked potential (MEP) and magnetic resonance imaging (MRI) were used to evaluate the integrity of the cerebral cortex. The neurologic score, motion capture, surface electromyography (sEMG), and vertical ground reaction force (vGRF) were measured in the analysis of motor functions. In vitro analysis of inflammation levels was performed by Elisa while immunohistochemistry was used in track the fate of hUCMSCs. In situ hybridization, transmission electron microscope, and immunofluorescence were used to assess neural and vascular regeneration. Results: Favorable physical properties, suitable degradation rate, and biocompatibility were observed in the collagen/SF scaffolds. The group with complex implantation exhibited the best cerebral cortex integrity and motor functions. The implantation also led to the regeneration of more blood vessels and nerve fibers, less glial fibers, and inflammatory factors. Conclusion: Implantation of this complex enhanced therapy in traumatic brain injury (TBI) through structural repair and functional recovery. These effects exhibit the translational prospects for the clinical application of this complex.
Keywords: Collagen; Hemiplegic limb; Mesenchymal stem cell; Silk fibroin; Traumatic brain injury.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
Recovery of motor function in rats with complete spinal cord injury following implantation of collagen/silk fibroin scaffold combined with human umbilical cord-mesenchymal stem cells.Rev Assoc Med Bras (1992). 2021 Sep;67(9):1342-1348. doi: 10.1590/1806-9282.20200697. Rev Assoc Med Bras (1992). 2021. PMID: 34816932
-
3D printing of injury-preconditioned secretome/collagen/heparan sulfate scaffolds for neurological recovery after traumatic brain injury in rats.Stem Cell Res Ther. 2022 Dec 19;13(1):525. doi: 10.1186/s13287-022-03208-0. Stem Cell Res Ther. 2022. PMID: 36536463 Free PMC article.
-
3D printing collagen/heparin sulfate scaffolds boost neural network reconstruction and motor function recovery after traumatic brain injury in canine.Biomater Sci. 2020 Nov 21;8(22):6362-6374. doi: 10.1039/d0bm01116a. Epub 2020 Oct 7. Biomater Sci. 2020. PMID: 33026366
-
Can Mesenchymal Stem Cells Act Multipotential in Traumatic Brain Injury?J Mol Neurosci. 2020 May;70(5):677-688. doi: 10.1007/s12031-019-01475-w. Epub 2020 Jan 2. J Mol Neurosci. 2020. PMID: 31897971 Review.
-
The use of bioactive matrices in regenerative therapies for traumatic brain injury.Acta Biomater. 2020 Jan 15;102:1-12. doi: 10.1016/j.actbio.2019.11.032. Epub 2019 Nov 18. Acta Biomater. 2020. PMID: 31751809 Review.
Cited by
-
Heterogeneity of In Vitro Expanded Mesenchymal Stromal Cells and Strategies to Improve Their Therapeutic Actions.Pharmaceutics. 2022 May 23;14(5):1112. doi: 10.3390/pharmaceutics14051112. Pharmaceutics. 2022. PMID: 35631698 Free PMC article. Review.
-
Hypoxia-pretreated mesenchymal stem cell-derived exosomes-loaded low-temperature extrusion 3D-printed implants for neural regeneration after traumatic brain injury in canines.Front Bioeng Biotechnol. 2022 Sep 30;10:1025138. doi: 10.3389/fbioe.2022.1025138. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36246376 Free PMC article.
-
Application and prospects of high-throughput screening for in vitro neurogenesis.World J Stem Cells. 2022 Jun 26;14(6):393-419. doi: 10.4252/wjsc.v14.i6.393. World J Stem Cells. 2022. PMID: 35949394 Free PMC article. Review.
-
Oriented artificial niche provides physical-biochemical stimulations for rapid nerve regeneration.Mater Today Bio. 2023 Jul 20;22:100736. doi: 10.1016/j.mtbio.2023.100736. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37521524 Free PMC article.
-
Silk Fibroin Materials: Biomedical Applications and Perspectives.Bioengineering (Basel). 2024 Feb 9;11(2):167. doi: 10.3390/bioengineering11020167. Bioengineering (Basel). 2024. PMID: 38391652 Free PMC article. Review.
References
-
- Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A. et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. - PubMed
-
- Jiang J, Gao G, Feng J, Mao Q, Chen L, Yang X. et al. Traumatic brain injury in China. Lancet Neurol. 2019;18:286–95. - PubMed
-
- Ma Y, Zeng X, Qiu X, Wei Q, Che M, Ding Y. et al. Perineurium-like sheath derived from long-term surviving mesenchymal stem cells confers nerve protection to the injured spinal cord. Biomaterials. 2018;160:37–55. - PubMed
-
- Liu Z, Tang M, Zhao J, Chai R, Kang J. Looking into the Future: Toward Advanced 3D Biomaterials for Stem-Cell-Based Regenerative Medicine. Advanced materials (Deerfield Beach, Fla.) 2018;30:e1705388–1705388e1705388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

