M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice
- PMID: 33391532
- PMCID: PMC7738903
- DOI: 10.7150/thno.48761
M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice
Abstract
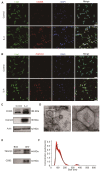
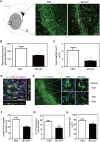
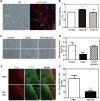
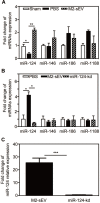
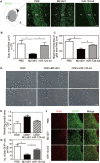
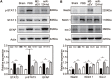
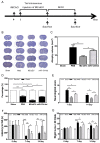
Rationale: Glial scars present a major obstacle for neuronal regeneration after stroke. Thus, approaches to promote their degradation and inhibit their formation are beneficial for stroke recovery. The interaction of microglia and astrocytes is known to be involved in glial scar formation after stroke; however, how microglia affect glial scar formation remains unclear. Methods: Mice were treated daily with M2 microglial small extracellular vesicles through tail intravenous injections from day 1 to day 7 after middle cerebral artery occlusion. Glial scar, infarct volume, neurological score were detected after ischemia. microRNA and related protein were examined in peri-infarct areas of the brain following ischemia. Results: M2 microglial small extracellular vesicles reduced glial scar formation and promoted recovery after stroke and were enriched in miR-124. Furthermore, M2 microglial small extracellular vesicle treatment decreased the expression of the astrocyte proliferation gene signal transducer and activator of transcription 3, one of the targets of miR-124, and glial fibrillary acidic protein and inhibited astrocyte proliferation both in vitro and in vivo. It also decreased Notch 1 expression and increased Sox2 expression in astrocytes, which suggested that astrocytes had transformed into neuronal progenitor cells. Finally, miR-124 knockdown in M2 microglial small extracellular vesicles blocked their effects on glial scars and stroke recovery. Conclusions: Our results showed, for the first time, that microglia regulate glial scar formation via small extracellular vesicles, indicating that M2 microglial small extracellular vesicles could represent a new therapeutic approach for stroke.
Keywords: astrocyte; glial scar; ischemic stroke; microglia; small extracellular vesicles.
© The author(s).
Conflict of interest statement
Competing Interests: The authors declare no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Figures

References
-
- Koyama Y. Signaling molecules regulating phenotypic conversions of astrocytes and glial scar formation in damaged nerve tissues. Neurochem Int. 2014;78:35–42. - PubMed
-
- Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z. et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870–86. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

