The potential role of leukoaraiosis in remodeling the brain network to buffer cognitive decline: a Leukoaraiosis And Disability study from Alzheimer's Disease Neuroimaging Initiative
- PMID: 33392021
- PMCID: PMC7719947
- DOI: 10.21037/qims-20-580
The potential role of leukoaraiosis in remodeling the brain network to buffer cognitive decline: a Leukoaraiosis And Disability study from Alzheimer's Disease Neuroimaging Initiative
Abstract
Background: Leukoaraiosis (LA) is a phenomenon of the brain that is often observed in elderly people. However, little is known about the role of LA in cognitive impairment in neurodegeneration and disease. This cross-sectional, retrospective Leukoaraiosis And Disability (LADIS) study aimed to characterize the relationship between brain white matter connectivity properties with LA ratings in patients with Alzheimer's disease (AD) as compared with age-matched cognitively normal controls.
Methods: Patients with AD (n=76) and elderly individuals with normal cognitive (NC) function (n=82) were classified into 3 groups, LA1, LA2, and LA3, according to the rating of their white matter changes (WMCs). Diffusion tensor imaging (DTI) data were analyzed by quantifying and comparing the white matter connectivity properties and gray matter (GM) volume of brain regions of interest (ROIs).
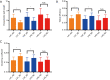
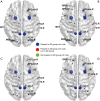
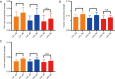
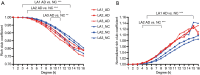
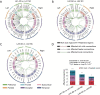
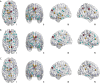
Results: The rich-club network properties in the AD LA1 and LA2 groups showed significant patterns of disrupted peripheral regions and reduced connectivity compared to those in the NC LA1 and LA2 groups, respectively. However, the rich-club network properties in the AD LA3 group showed similar patterns of disrupted peripheral regions and reduced connectivity compared to those in the NC LA3 group, despite there being significant hippocampal and amygdala atrophic differences between AD patients and NC elders. Compared to the NC LA1 group, the characteristic path length of white matter fiber connectivity in the NC LA3 group was significantly increased, and the brain's global efficiency, clustering coefficient, and network connectivity strength were significantly reduced (P<0.05, respectively). However, no significant differences (P>0.05) were observed in characteristic path length, reduced global efficiency, or the clustering coefficient between the NC LA3 and AD LA1 groups, or between the NC LA3 and AD LA2 groups.
Conclusions: Our findings offer some insights into a potential role of LA in cognitive impairment that may predict the development of disability in older adults. The occurrence of LA, an intermediate degenerative change, during neurodegeneration and disease may potentially lead to the remodeling of the brain network through brain plasticity. LA, therefore, representing a possible compensatory mechanism to buffer cognitive decline.
Keywords: White matter hyperintensities (WMH); brain plasticity; network-based statistic (NBS); quantitative magnetic resonance imaging (qMRI); rich club; voxel-based morphometry (VBM).
2021 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-580). The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Structural Alteration of Medial Temporal Lobe Subfield in the Amnestic Mild Cognitive Impairment Stage of Alzheimer's Disease.Neural Plast. 2022 Jan 24;2022:8461235. doi: 10.1155/2022/8461235. eCollection 2022. Neural Plast. 2022. PMID: 35111220 Free PMC article.
-
Effects of Brain Parcellation on the Characterization of Topological Deterioration in Alzheimer's Disease.Front Aging Neurosci. 2019 May 21;11:113. doi: 10.3389/fnagi.2019.00113. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31164815 Free PMC article.
-
Rich club analysis in the Alzheimer's disease connectome reveals a relatively undisturbed structural core network.Hum Brain Mapp. 2015 Aug;36(8):3087-103. doi: 10.1002/hbm.22830. Epub 2015 Jun 3. Hum Brain Mapp. 2015. PMID: 26037224 Free PMC article.
-
Rich-club in the brain's macrostructure: Insights from graph theoretical analysis.Comput Struct Biotechnol J. 2020 Jun 29;18:1761-1773. doi: 10.1016/j.csbj.2020.06.039. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32695269 Free PMC article. Review.
-
Use of Multimodal Magnetic Resonance Imaging Techniques to Explore Cognitive Impairment in Leukoaraiosis.Med Sci Monit. 2018 Dec 9;24:8910-8915. doi: 10.12659/MSM.912153. Med Sci Monit. 2018. PMID: 30531675 Free PMC article. Review.
Cited by
-
Dynamic functional connections in leukoaraiosis patients without cognitive impairment: A pilot study.Front Aging Neurosci. 2022 Sep 1;14:944485. doi: 10.3389/fnagi.2022.944485. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36118700 Free PMC article.
-
The characteristic patterns of individual brain susceptibility networks underlie Alzheimer's disease and white matter hyperintensity-related cognitive impairment.Transl Psychiatry. 2024 Apr 4;14(1):177. doi: 10.1038/s41398-024-02861-8. Transl Psychiatry. 2024. PMID: 38575556 Free PMC article.
-
Evaluating white matter alterations in Parkinson's disease-related parkin S/N167 mutation carriers using tract-based spatial statistics.Quant Imaging Med Surg. 2022 Aug;12(8):4272-4285. doi: 10.21037/qims-21-1007. Quant Imaging Med Surg. 2022. PMID: 35919057 Free PMC article.
References
-
- Mills SE. Histology for Pathologists. Philadelphia: Lippincott Williams & Wilkins, 2012:301-2.
-
- van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain 1991;114:761-74. 10.1093/brain/114.2.761 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
