VARP and Rab9 Are Dispensable for the Rab32/BLOC-3 Dependent Salmonella Killing
- PMID: 33392103
- PMCID: PMC7772198
- DOI: 10.3389/fcimb.2020.581024
VARP and Rab9 Are Dispensable for the Rab32/BLOC-3 Dependent Salmonella Killing
Abstract
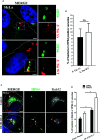
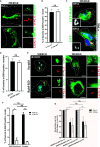
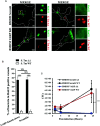
Salmonella enterica serovar Typhi (S. Typhi) is the causative agent of typhoid fever, a disease that kills an estimated 200,000 people annually. Previously, we discovered an antimicrobial pathway dependent on Rab32 and BLOC-3 (BRAM) that is critical to kill S. Typhi in murine macrophages. The BLOC-3 complex is comprised of the two sub-units HPS1 and HPS4 and exhibits guanine-nucleotide exchange factor (GEF) activity to Rab32. In melanocytes, Rab9 has been shown to interact with HPS4 and RUTBC1, a Rab32 GTPase activating (GAP) protein, and regulate the Rab32-mediated melanosome biogenesis. Intriguingly, Rab9-deficient melanocytes exhibit hypopigmentation, a similar phenotype to Rab32 or BLOC-3 deficient melanocytes. Additionally, VPS9-ankyrin-repeat-protein (VARP) has been shown to regulate melanocytic enzyme trafficking into the melanosomes through interaction with Rab32. Although Rab32, Rab9 and VARP are a part of melanogenesis in melanocytes, whether Rab9 and VARP are required for the BRAM mediated killing in macrophages is currently unknown. Here we showed that HPS4 is recruited to the Salmonella-containing vacuoles (SCV) and over-expression of BLOC-3 significantly increased Rab32-positive bacteria vacuoles. We found that SCV acquire Rab9, however over-expressing Rab9 did not change HPS4 localization on bacteria vacuoles. Importantly, we used shRNA to knock-down Rab9 and VARP in macrophages and showed that these proteins are dispensable for Rab32 recruitment to the SCV. Furthermore, we assessed the survival of S. Typhimurium in macrophages deficient for Rab9 or VARP and demonstrated that these proteins are not essential for BRAM pathway-dependent killing.
Keywords: BLOC-3; Rab32; Rab9; Salmonella; VPS9-ankyrin-repeat-protein; macrophages.
Copyright © 2020 Balci, Solano-Collado, Baldassarre and Spanò.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The BLOC-3 subunit HPS4 is required for activation of Rab32/38 GTPases in melanogenesis, but its Rab9 activity is dispensable for melanogenesis.J Biol Chem. 2019 Apr 26;294(17):6912-6922. doi: 10.1074/jbc.RA119.007345. Epub 2019 Mar 5. J Biol Chem. 2019. PMID: 30837268 Free PMC article.
-
Structure-function analysis of VPS9-ankyrin-repeat protein (Varp) in the trafficking of tyrosinase-related protein 1 in melanocytes.J Biol Chem. 2011 Mar 4;286(9):7507-21. doi: 10.1074/jbc.M110.191205. Epub 2010 Dec 26. J Biol Chem. 2011. PMID: 21187289 Free PMC article.
-
The Rab21-GEF activity of Varp, but not its Rab32/38 effector function, is required for dendrite formation in melanocytes.Mol Biol Cell. 2012 Feb;23(4):669-78. doi: 10.1091/mbc.E11-04-0324. Epub 2011 Dec 14. Mol Biol Cell. 2012. PMID: 22171327 Free PMC article.
-
Multiple Roles of VARP in Endosomal Trafficking: Rabs, Retromer Components and R-SNARE VAMP7 Meet on VARP.Traffic. 2016 Jul;17(7):709-19. doi: 10.1111/tra.12406. Epub 2016 May 13. Traffic. 2016. PMID: 27103185 Review.
-
Mechanisms of Salmonella Typhi Host Restriction.Adv Exp Med Biol. 2016;915:283-94. doi: 10.1007/978-3-319-32189-9_17. Adv Exp Med Biol. 2016. PMID: 27193549 Review.
Cited by
-
Cryo-EM structure of the BLOC-3 complex provides insights into the pathogenesis of Hermansky-Pudlak syndrome.Nat Commun. 2025 Mar 26;16(1):2967. doi: 10.1038/s41467-025-58235-1. Nat Commun. 2025. PMID: 40140412 Free PMC article.
-
Dot/Icm-Dependent Restriction of Legionella pneumophila within Neutrophils.mBio. 2021 Jun 29;12(3):e0100821. doi: 10.1128/mBio.01008-21. Epub 2021 May 26. mBio. 2021. PMID: 34076467 Free PMC article.
-
Mitochondrial A-kinase anchoring proteins in cardiac ventricular myocytes.Physiol Rep. 2021 Sep;9(17):e15015. doi: 10.14814/phy2.15015. Physiol Rep. 2021. PMID: 34514737 Free PMC article.
-
Hermansky-Pudlak syndrome type 1 causes impaired anti-microbial immunity and inflammation due to dysregulated immunometabolism.Mucosal Immunol. 2022 Jun;15(6):1431-1446. doi: 10.1038/s41385-022-00572-1. Epub 2022 Oct 27. Mucosal Immunol. 2022. PMID: 36302964 Free PMC article.
-
Rab32 family proteins regulate autophagosomal components recycling.J Cell Biol. 2024 Mar 4;223(3):e202306040. doi: 10.1083/jcb.202306040. Epub 2024 Feb 7. J Cell Biol. 2024. PMID: 38323995 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

