Enteropathogenic Escherichia coli Infection Induces Diarrhea, Intestinal Damage, Metabolic Alterations, and Increased Intestinal Permeability in a Murine Model
- PMID: 33392105
- PMCID: PMC7773950
- DOI: 10.3389/fcimb.2020.595266
Enteropathogenic Escherichia coli Infection Induces Diarrhea, Intestinal Damage, Metabolic Alterations, and Increased Intestinal Permeability in a Murine Model
Abstract
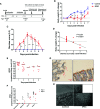
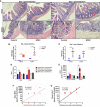
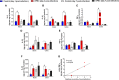
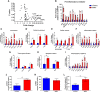
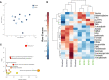
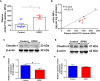
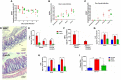
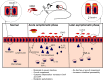
Enteropathogenic E. coli (EPEC) are recognized as one of the leading bacterial causes of infantile diarrhea worldwide. Weaned C57BL/6 mice pretreated with antibiotics were challenged orally with wild-type EPEC or escN mutant (lacking type 3 secretion system) to determine colonization, inflammatory responses and clinical outcomes during infection. Antibiotic disruption of intestinal microbiota enabled efficient colonization by wild-type EPEC resulting in growth impairment and diarrhea. Increase in inflammatory biomarkers, chemokines, cellular recruitment and pro-inflammatory cytokines were observed in intestinal tissues. Metabolomic changes were also observed in EPEC infected mice with changes in tricarboxylic acid (TCA) cycle intermediates, increased creatine excretion and shifts in gut microbial metabolite levels. In addition, by 7 days after infection, although weights were recovering, EPEC-infected mice had increased intestinal permeability and decreased colonic claudin-1 levels. The escN mutant colonized the mice with no weight loss or increased inflammatory biomarkers, showing the importance of the T3SS in EPEC virulence in this model. In conclusion, a murine infection model treated with antibiotics has been developed to mimic clinical outcomes seen in children with EPEC infection and to examine potential roles of selected virulence traits. This model can help in further understanding mechanisms involved in the pathogenesis of EPEC infections and potential outcomes and thus assist in the development of potential preventive or therapeutic interventions.
Keywords: antibiotics; diarrhea; enteropathogenic Escherichia coli; enteropathy; inflammation; murine model.
Copyright © 2020 Ledwaba, Costa, Bolick, Giallourou, Medeiros, Swann, Traore, Potgieter, Nataro and Guerrant.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Enteropathogenic Escherichia coli (EPEC) expressing a non-functional bundle-forming pili (BFP) also leads to increased growth failure and intestinal inflammation in C57BL/6 mice.Braz J Microbiol. 2022 Dec;53(4):1781-1787. doi: 10.1007/s42770-022-00802-5. Epub 2022 Jul 26. Braz J Microbiol. 2022. PMID: 35882715 Free PMC article.
-
Oral infection with enteropathogenic Escherichia coli triggers immune response and intestinal histological alterations in mice selected for their minimal acute inflammatory responses.Microbiol Immunol. 2014 Jun;58(6):352-9. doi: 10.1111/1348-0421.12153. Microbiol Immunol. 2014. PMID: 24750489
-
Escherichia coli B2 Phylogenetic Subgroups in the Infant Gut Microbiota: Predominance of Uropathogenic Lineages in Swedish Infants and Enteropathogenic Lineages in Pakistani Infants.Appl Environ Microbiol. 2019 Nov 27;85(24):e01681-19. doi: 10.1128/AEM.01681-19. Print 2019 Dec 15. Appl Environ Microbiol. 2019. PMID: 31562173 Free PMC article.
-
[Molecular pathogenesis, epidemiology and diagnosis of enteropathogenic Escherichia coli].Salud Publica Mex. 2007 Sep-Oct;49(5):376-86. doi: 10.1590/s0036-36342007000500008. Salud Publica Mex. 2007. PMID: 17952245 Review. Spanish.
-
Pathophysiology of enteropathogenic Escherichia coli during a host infection.J Vet Sci. 2022 Mar;23(2):e28. doi: 10.4142/jvs.21160. Epub 2022 Jan 27. J Vet Sci. 2022. PMID: 35187883 Free PMC article. Review.
Cited by
-
The synergistic interaction of systemic inflammation, dysbiosis and antimicrobial resistance promotes growth restriction in children with acute severe malnutrition: An emphasis on Escherichia coli.Front Antibiot. 2022 Oct 24;1:1001717. doi: 10.3389/frabi.2022.1001717. eCollection 2022. Front Antibiot. 2022. PMID: 39816412 Free PMC article. Review.
-
Mouse models for bacterial enteropathogen infections: insights into the role of colonization resistance.Gut Microbes. 2023 Jan-Dec;15(1):2172667. doi: 10.1080/19490976.2023.2172667. Gut Microbes. 2023. PMID: 36794831 Free PMC article.
-
Prophylactic Catechin-Rich Green Tea Extract Treatment Ameliorates Pathogenic Enterotoxic Escherichia coli-Induced Colitis.Pathogens. 2021 Dec 2;10(12):1573. doi: 10.3390/pathogens10121573. Pathogens. 2021. PMID: 34959529 Free PMC article.
-
Antimicrobial Effects of Kelisha Capsule on Escherichia coli Induced Diarrhea in Mice.Drug Des Devel Ther. 2025 Apr 10;19:2779-2794. doi: 10.2147/DDDT.S511158. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40231195 Free PMC article.
-
Plasmon-Enhanced Antibacterial Activity of Chiral Gold Nanoparticles and In Vivo Therapeutic Effect.Nanomaterials (Basel). 2021 Jun 21;11(6):1621. doi: 10.3390/nano11061621. Nanomaterials (Basel). 2021. PMID: 34205616 Free PMC article.
References
-
- Abe C. M., Trabulsi L. R., Blanco J., Blanco M., Dahbi G., Blanco J. E., et al. (2009). Virulence features of atypical enteropathogenic Escherichia coli identified by the eae+ EAF-negative stx– genetic profile. Diagn. Microbiol. Infect. Dis. 64 (4), 357–365. 10.1016/j.diagmicrobio.2009.03.025 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

