Salmonella Effector SpvB Disrupts Intestinal Epithelial Barrier Integrity for Bacterial Translocation
- PMID: 33392110
- PMCID: PMC7773751
- DOI: 10.3389/fcimb.2020.606541
Salmonella Effector SpvB Disrupts Intestinal Epithelial Barrier Integrity for Bacterial Translocation
Abstract
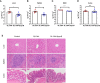
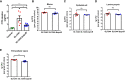
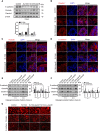
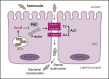
Salmonella are common enteric bacterial pathogens that infect both humans and animals. Intestinal epithelial barrier, formed by a single layer of epithelial cells and apical junctional complex (AJC), plays a crucial role in host defense against enteric pathogens to prevent bacterial translocation. However, the underlying mechanisms of intestinal epithelial barrier dysfunction caused by Salmonella are poorly understood. It is found that a locus termed Salmonella plasmid virulence (spv) gene exists extensively in clinically important Salmonella serovars. SpvB is a key effector encoded within this locus, and closely related to Salmonella pathogenicity such as interfering with autophagy and iron homeostasis. To investigate the interaction between SpvB and intestinal epithelial barrier and elucidate the underlying molecular mechanism, we used the typical foodborne disease agent Salmonella enterica serovar Typhimurium (Salmonella typhimurium) carrying spvB or not to construct infection models in vivo and in vitro. C57BL/6 mice were orally challenged with S. typhimurium wild-type strain SL1344 or spvB-deficient mutant strain SL1344-ΔspvB. Caco-2 cell monolayer model, as a widely used model to mimic the human intestinal epithelium in vitro, was infected with SL1344, SL1344-ΔspvB, or spvB complementary strain SL1344-c-ΔspvB, respectively. The results showed that SpvB enhanced bacterial pathogenicity during S. typhimurium infection in vivo, and contributed to intestinal epithelial barrier dysfunction in both infection systems. This SpvB-mediated barrier dysfunction was attributed to the cellular redistribution of Claudin-1, Occludin, and E-cadherin junctional proteins. Moreover, by using pharmacological inhibitors, we found that F-actin rearrangement and suppression of protein kinase C (PKC) signaling pathway were involved in SpvB-mediated barrier dysfunction. In conclusion, the study reveals the contribution of Salmonella effector SpvB to the dysfunction of intestinal epithelial barrier integrity, which facilitates bacterial translocation via the paracellular route to promote Salmonella systemic dissemination. Our findings broaden the understanding of host-pathogen interactions in salmonellosis, and provide new strategies for the therapy in limiting bacterial dissemination during infection.
Keywords: F-actin; Salmonella; SpvB; apical junctional complex; intestinal epithelial barrier; protein kinase C.
Copyright © 2020 Sun, Yang, Deng, Dong, Li, Wu and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Salmonella pSLT-encoded effector SpvB promotes RIPK3-dependent necroptosis in intestinal epithelial cells.Cell Death Discov. 2022 Feb 2;8(1):44. doi: 10.1038/s41420-022-00841-9. Cell Death Discov. 2022. PMID: 35110556 Free PMC article.
-
Salmonella plasmid virulence gene spvB enhances bacterial virulence by inhibiting autophagy in a zebrafish infection model.Fish Shellfish Immunol. 2016 Feb;49:252-9. doi: 10.1016/j.fsi.2015.12.033. Epub 2015 Dec 23. Fish Shellfish Immunol. 2016. PMID: 26723267
-
A novel contribution of spvB to pathogenesis of Salmonella Typhimurium by inhibiting autophagy in host cells.Oncotarget. 2016 Feb 16;7(7):8295-309. doi: 10.18632/oncotarget.6989. Oncotarget. 2016. PMID: 26811498 Free PMC article.
-
The role of host cell death in Salmonella infections.Curr Top Microbiol Immunol. 2005;289:131-50. doi: 10.1007/3-540-27320-4_6. Curr Top Microbiol Immunol. 2005. PMID: 15791954 Review.
-
New insights into the mode of action of the actin ADP-ribosylating virulence factors Salmonella enterica SpvB and Clostridium botulinum C2 toxin.Eur J Cell Biol. 2011 Nov;90(11):944-50. doi: 10.1016/j.ejcb.2010.11.007. Epub 2011 Jan 17. Eur J Cell Biol. 2011. PMID: 21247657 Review.
Cited by
-
Type 3 immune response protects against Salmonella Typhimurium infection in the small intestine of neonatal rats.Emerg Microbes Infect. 2024 Dec;13(1):2417867. doi: 10.1080/22221751.2024.2417867. Epub 2024 Oct 28. Emerg Microbes Infect. 2024. PMID: 39435479 Free PMC article.
-
Protective Capacity of Helichrysum italicum Infusion Against Intestinal Barrier Disruption and Translocation of Salmonella Infantis.Pharmaceuticals (Basel). 2024 Oct 19;17(10):1398. doi: 10.3390/ph17101398. Pharmaceuticals (Basel). 2024. PMID: 39459037 Free PMC article.
-
Salmonella pSLT-encoded effector SpvB promotes RIPK3-dependent necroptosis in intestinal epithelial cells.Cell Death Discov. 2022 Feb 2;8(1):44. doi: 10.1038/s41420-022-00841-9. Cell Death Discov. 2022. PMID: 35110556 Free PMC article.
-
Microbiota as the unifying factor behind the hallmarks of cancer.J Cancer Res Clin Oncol. 2023 Nov;149(15):14429-14450. doi: 10.1007/s00432-023-05244-6. Epub 2023 Aug 9. J Cancer Res Clin Oncol. 2023. PMID: 37555952 Free PMC article. Review.
-
Probiotic Bacteria and Their Cell Walls Induce Th1-Type Immunity Against Salmonella Typhimurium Challenge.Front Immunol. 2021 May 12;12:660854. doi: 10.3389/fimmu.2021.660854. eCollection 2021. Front Immunol. 2021. PMID: 34054825 Free PMC article.
References
-
- Bou Ghanem E. N., Jones G. S., Myers-Morales T., Patil P. D., Hidayatullah A. N., D’orazio S. E. (2012). InlA promotes dissemination of Listeria monocytogenes to the mesenteric lymph nodes during food borne infection of mice. PloS Pathog 8, e1003015. 10.1371/journal.ppat.1003015 - DOI - PMC - PubMed
-
- Buckner M. M., Croxen M. A., Arena E. T., Finlay B. B. (2011). A comprehensive study of the contribution of Salmonella enterica serovar Typhimurium SPI2 effectors to bacterial colonization, survival, and replication in typhoid fever, macrophage, and epithelial cell infection models. Virulence 2, 208–216. 10.4161/viru.2.3.15894 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

