Necator americanus Ancylostoma Secreted Protein-2 (Na-ASP-2) Binds an Ascaroside (ascr#3) in Its Fatty Acid Binding Site
- PMID: 33392151
- PMCID: PMC7773830
- DOI: 10.3389/fchem.2020.608296
Necator americanus Ancylostoma Secreted Protein-2 (Na-ASP-2) Binds an Ascaroside (ascr#3) in Its Fatty Acid Binding Site
Abstract
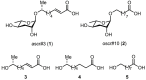
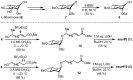
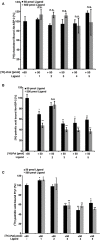
During their infective stages, hookworms release excretory-secretory (E-S) products, small molecules, and proteins to help evade and suppress the host's immune system. Small molecules found in E-S products of mammalian hookworms include nematode derived metabolites like ascarosides, which are composed of the sugar ascarylose linked to a fatty acid side chain. The most abundant proteins found in hookworm E-S products are members of the protein family known as Ancylostoma secreted protein (ASP). In this study, two ascarosides and their fatty acid moieties were synthesized and tested for in vitro binding to Na-ASP-2 using both a ligand competition assay and microscale thermophoresis. The fatty acid moieties of both ascarosides tested and ascr#3, an ascaroside found in rat hookworm E-S products, bind to Na-ASP-2's palmitate binding cavity. These molecules were confirmed to bind to the palmitate but not the sterol binding sites. An ascaroside, oscr#10, which is not found in hookworm E-S products, does not bind to Na-ASP-2. More studies are required to determine the structural basis of ascarosides binding by Na-ASP-2 and to understand the physiological significance of these observations.
Keywords: CAP [cysteine-rich secretory protein (CRISP)/antigen 5/pathogenesis related-1 (PR-1)]; TAPs [testis specific proteins (Tpx)/antigen 5 (Ag5)/pathogenesis related-1 (PR-1)/Sc7]; lipid binding; sperm coating protein (SCP); venom allergen-like (VAL).
Copyright © 2020 El Atab, Darwiche, Truax, Schneiter, Hull, Romo and Asojo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Asojo O. A., Goud G., Dhar K., Loukas A., Zhan B., Deumic V., et al. . (2005). X-ray structure of Na-ASP-2, a pathogenesis-related-1 protein from the nematode parasite, necator americanus, and a vaccine antigen for human hookworm infection. J. Mol. Biol. 346, 801–814. 10.1016/j.jmb.2004.12.023 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

