The Impact of Biomaterial Cell Contact on the Immunopeptidome
- PMID: 33392160
- PMCID: PMC7773052
- DOI: 10.3389/fbioe.2020.571294
The Impact of Biomaterial Cell Contact on the Immunopeptidome
Abstract
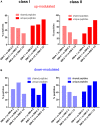
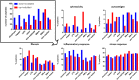
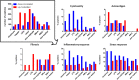
Biomaterials play an increasing role in clinical applications and regenerative medicine. A perfectly designed biomaterial should restore the function of damaged tissue without triggering an undesirable immune response, initiate self-regeneration of the surrounding tissue and gradually degrade after implantation. The immune system is well recognized to play a major role in influencing the biocompatibility of implanted medical devices. To obtain a better understanding of the effects of biomaterials on the immune response, we have developed a highly sensitive novel test system capable of examining changes in the immune system by biomaterial. Here, we evaluated for the first time the immunopeptidome, a highly sensitive system that reflects cancer transformation, virus or drug influences and passes these cellular changes directly to T cells, as a test system to examine the effects of contact with materials. Since monocytes are one of the first immune cells reacting to biomaterials, we have tested the influence of different materials on the immunopeptidome of the monocytic THP-1 cell line. The tested materials included stainless steel, aluminum, zinc, high-density polyethylene, polyurethane films containing zinc diethyldithiocarbamate, copper, and zinc sulfate. The incubation with all material types resulted in significantly modulated peptides in the immunopeptidome, which were material-associated. The magnitude of induced changes in the immunopeptidome after the stimulation appeared comparable to that of bacterial lipopolysaccharides (LPS). The source proteins of many detected peptides are associated with cytotoxicity, fibrosis, autoimmunity, inflammation, and cellular stress. Considering all tested materials, it was found that the LPS-induced cytotoxicity-, inflammation- and cellular stress-associated HLA class I peptides were mainly induced by aluminum, whereas HLA class II peptides were mainly induced by stainless steel. These findings provide the first insights into the effects of biomaterials on the immunopeptidome. A more thorough understanding of these effects may enable the design of more biocompatible implant materials using in vitro models in future. Such efforts will provide a deeper understanding of possible immune responses induced by biomaterials such as fibrosis, inflammation, cytotoxicity, and autoimmune reactions.
Keywords: biocompatibility; biocompatibility assay; biomaterials; foreign body response; host response; immunopeptidomics; mass spectrometry; monocytes.
Copyright © 2020 Ghosh, Hartmann, Jakobi, März, Bichmann, Freudenmann, Mühlenbruch, Segan, Rammensee, Schneiderhan-Marra, Shipp, Stevanović and Joos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Anderson J. M. (1993). Chapter 4 Mechanisms of inflammation and infection with implanted devices. Cardiovasc. Pathol. 2 33–41. 10.1016/1054-8807(93)90045-4 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

