Does Blast Exposure to the Torso Cause a Blood Surge to the Brain?
- PMID: 33392161
- PMCID: PMC7773947
- DOI: 10.3389/fbioe.2020.573647
Does Blast Exposure to the Torso Cause a Blood Surge to the Brain?
Abstract
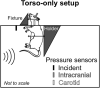
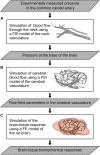
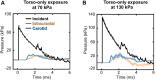
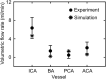
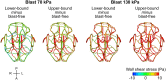
The interaction of explosion-induced blast waves with the torso is suspected to contribute to brain injury. In this indirect mechanism, the wave-torso interaction is assumed to generate a blood surge, which ultimately reaches and damages the brain. However, this hypothesis has not been comprehensively and systematically investigated, and the potential role, if any, of the indirect mechanism in causing brain injury remains unclear. In this interdisciplinary study, we performed experiments and developed mathematical models to address this knowledge gap. First, we conducted blast-wave exposures of Sprague-Dawley rats in a shock tube at incident overpressures of 70 and 130 kPa, where we measured carotid-artery and brain pressures while limiting exposure to the torso. Then, we developed three-dimensional (3-D) fluid-structure interaction (FSI) models of the neck and cerebral vasculature and, using the measured carotid-artery pressures, performed simulations to predict mass flow rates and wall shear stresses in the cerebral vasculature. Finally, we developed a 3-D finite element (FE) model of the brain and used the FSI-computed vasculature pressures to drive the FE model to quantify the blast-exposure effects in the brain tissue. The measurements from the torso-only exposure experiments revealed marginal increases in the peak carotid-artery overpressures (from 13.1 to 28.9 kPa). Yet, relative to the blast-free, normotensive condition, the FSI simulations for the blast exposures predicted increases in the peak mass flow rate of up to 255% at the base of the brain and increases in the wall shear stress of up to 289% on the cerebral vasculature. In contrast, our simulations suggest that the effect of the indirect mechanism on the brain-tissue-strain response is negligible (<1%). In summary, our analyses show that the indirect mechanism causes a sudden and abundant stream of blood to rapidly propagate from the torso through the neck to the cerebral vasculature. This blood surge causes a considerable increase in the wall shear stresses in the brain vasculature network, which may lead to functional and structural effects on the cerebral veins and arteries, ultimately leading to vascular pathology. In contrast, our findings do not support the notion of strain-induced brain-tissue damage due to the indirect mechanism.
Keywords: blast overpressure; fluid-structure interaction; indirect mechanism; shock tube; traumatic brain injury.
Copyright © 2020 Rubio, Skotak, Alay, Sundaramurthy, Subramaniam, Kote, Yeoh, Monson, Chandra, Unnikrishnan and Reifman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alay E., Skotak M., Misistia A., Chandra N. (2018). Dynamic loads on human and animal surrogates at different test locations in compressed-gas-driven shock tubes. Shock Waves 28 51–62. 10.1007/s00193-017-0762-4 - DOI
-
- Ambrosi D., Quarteroni A., Rozza G. (2012). Modeling of Physiological Flows. Milan: Springer.
-
- Aoki T., Nishimura M., Matsuoka T., Yamamoto K., Furuyashiki T., Kataoka H., et al. (2011). PGE(2) -EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br. J. Pharmacol. 163 1237–1249. 10.1111/j.1476-5381.2011.01358.x - DOI - PMC - PubMed
-
- Assari S., Laksari K., Barbe M., Darvish K. (2013). “Cerebral blood pressure rise during blast exposure in a rat model of blast-induced traumatic brain injury,” in Proceedings of the ASME 2013 International Mechanical Engineering Congress and Exposition, (V03AT03A016) (San Diego, CA: ).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

