Applicability of Recombinant Laccases From the White-Rot Fungus Obba rivulosa for Mediator-Promoted Oxidation of Biorefinery Lignin at Low pH
- PMID: 33392170
- PMCID: PMC7773891
- DOI: 10.3389/fbioe.2020.604497
Applicability of Recombinant Laccases From the White-Rot Fungus Obba rivulosa for Mediator-Promoted Oxidation of Biorefinery Lignin at Low pH
Abstract
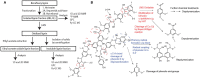
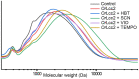
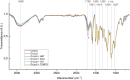
Utilization of lignin-rich side streams has been a focus of intensive studies recently. Combining biocatalytic methods with chemical treatments is a promising approach for sustainable modification of lignocellulosic waste streams. Laccases are catalysts in lignin biodegradation with proven applicability in industrial scale. Laccases directly oxidize lignin phenolic components, and their functional range can be expanded using low-molecular-weight compounds as mediators to include non-phenolic lignin structures. In this work, we studied in detail recombinant laccases from the selectively lignin-degrading white-rot fungus Obba rivulosa for their properties and evaluated their potential as industrial biocatalysts for the modification of wood lignin and lignin-like compounds. We screened and optimized various laccase mediator systems (LMSs) using lignin model compounds and applied the optimized reaction conditions to biorefinery-sourced technical lignin. In the presence of both N-OH-type and phenolic mediators, the O. rivulosa laccases were shown to selectively oxidize lignin in acidic reaction conditions, where a cosolvent is needed to enhance lignin solubility. In comparison to catalytic iron(III)-(2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) oxidation systems, the syringyl-type lignin units were preferred in mediated biocatalytic oxidation systems.
Keywords: acidic conditions; biorefinery lignin; laccase; mediator; oxidation; structural analysis.
Copyright © 2020 Kontro, Maltari, Mikkilä, Kähkönen, Mäkelä, Hildén, Nousiainen and Sipilä.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Depolymerization of biorefinery lignin by improved laccases of the white-rot fungus Obba rivulosa.Microb Biotechnol. 2021 Sep;14(5):2140-2151. doi: 10.1111/1751-7915.13896. Epub 2021 Jul 26. Microb Biotechnol. 2021. PMID: 34310858 Free PMC article.
-
Production of Recombinant Laccase From Coprinopsis cinerea and Its Effect in Mediator Promoted Lignin Oxidation at Neutral pH.Front Bioeng Biotechnol. 2021 Nov 9;9:767139. doi: 10.3389/fbioe.2021.767139. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34858962 Free PMC article.
-
Phenolic mediators enhance the manganese peroxidase catalyzed oxidation of recalcitrant lignin model compounds and synthetic lignin.Fungal Genet Biol. 2014 Nov;72:137-149. doi: 10.1016/j.fgb.2014.07.008. Epub 2014 Aug 7. Fungal Genet Biol. 2014. PMID: 25108071
-
Laccases for biorefinery applications: a critical review on challenges and perspectives.Bioprocess Biosyst Eng. 2015 Dec;38(12):2285-313. doi: 10.1007/s00449-015-1475-7. Epub 2015 Oct 5. Bioprocess Biosyst Eng. 2015. PMID: 26437966 Review.
-
Can laccases catalyze bond cleavage in lignin?Biotechnol Adv. 2015 Jan-Feb;33(1):13-24. doi: 10.1016/j.biotechadv.2014.12.008. Epub 2015 Jan 3. Biotechnol Adv. 2015. PMID: 25560931 Review.
Cited by
-
Depolymerization of biorefinery lignin by improved laccases of the white-rot fungus Obba rivulosa.Microb Biotechnol. 2021 Sep;14(5):2140-2151. doi: 10.1111/1751-7915.13896. Epub 2021 Jul 26. Microb Biotechnol. 2021. PMID: 34310858 Free PMC article.
-
Production of Recombinant Laccase From Coprinopsis cinerea and Its Effect in Mediator Promoted Lignin Oxidation at Neutral pH.Front Bioeng Biotechnol. 2021 Nov 9;9:767139. doi: 10.3389/fbioe.2021.767139. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34858962 Free PMC article.
-
A high-quality genome assembly of Lactarius hatsudake strain JH5.G3 (Bethesda). 2022 Dec 1;12(12):jkac262. doi: 10.1093/g3journal/jkac262. G3 (Bethesda). 2022. PMID: 36171643 Free PMC article.
-
Comparative Analysis of Enzyme Production Patterns of Lignocellulose Degradation of Two White Rot Fungi: Obba rivulosa and Gelatoporia subvermispora.Biomolecules. 2022 Jul 22;12(8):1017. doi: 10.3390/biom12081017. Biomolecules. 2022. PMID: 35892327 Free PMC article.
-
Endophytes in Lignin Valorization: A Novel Approach.Front Bioeng Biotechnol. 2022 Jul 19;10:895414. doi: 10.3389/fbioe.2022.895414. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35928943 Free PMC article. Review.
References
-
- Adler E., Lindgren B. O., Saedén U. (1952). The beta-guaiacyl ether of alpha-veratryl-glycerol as a lignin model. Sven. Papperstidning 55, 245–255.
-
- Balakshin M. Y., Capanema E. A. (2015). Comprehensive structural analysis of biorefinery lignins with a quantitative 13 C NMR approach. RSC Adv. 5, 87187–87199. 10.1039/C5RA16649G - DOI
LinkOut - more resources
Full Text Sources

