Perinatal Derivatives: Where Do We Stand? A Roadmap of the Human Placenta and Consensus for Tissue and Cell Nomenclature
- PMID: 33392174
- PMCID: PMC7773933
- DOI: 10.3389/fbioe.2020.610544
Perinatal Derivatives: Where Do We Stand? A Roadmap of the Human Placenta and Consensus for Tissue and Cell Nomenclature
Abstract
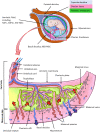
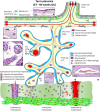
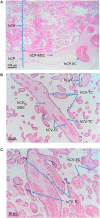
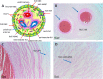
Progress in the understanding of the biology of perinatal tissues has contributed to the breakthrough revelation of the therapeutic effects of perinatal derivatives (PnD), namely birth-associated tissues, cells, and secreted factors. The significant knowledge acquired in the past two decades, along with the increasing interest in perinatal derivatives, fuels an urgent need for the precise identification of PnD and the establishment of updated consensus criteria policies for their characterization. The aim of this review is not to go into detail on preclinical or clinical trials, but rather we address specific issues that are relevant for the definition/characterization of perinatal cells, starting from an understanding of the development of the human placenta, its structure, and the different cell populations that can be isolated from the different perinatal tissues. We describe where the cells are located within the placenta and their cell morphology and phenotype. We also propose nomenclature for the cell populations and derivatives discussed herein. This review is a joint effort from the COST SPRINT Action (CA17116), which broadly aims at approaching consensus for different aspects of PnD research, such as providing inputs for future standards for the processing and in vitro characterization and clinical application of PnD.
Keywords: cells; consensus nomenclature; derivatives; fetal annexes; perinatal; placenta; tissues.
Copyright © 2020 Silini, Di Pietro, Lang-Olip, Alviano, Banerjee, Basile, Borutinskaite, Eissner, Gellhaus, Giebel, Huang, Janev, Kreft, Kupper, Abadía-Molina, Olivares, Pandolfi, Papait, Pozzobon, Ruiz-Ruiz, Soritau, Susman, Szukiewicz, Weidinger, Wolbank, Huppertz and Parolini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Abomaray F. M., Al Jumah M. A., Kalionis B., AlAskar A. S., Al Harthy S. (2015). Human chorionic villous mesenchymal stem cells modify the functions of human dendritic cells, and induce an anti-inflammatory phenotype in CD1+ dendritic cells. Stem Cell Rev. Rep. 11 423–441. 10.1007/s12015-014-9562-8 - DOI - PubMed
-
- Abumaree M. H., Abomaray F. M., Alshehri N. A., Almutairi A., AlAskar A. S., Al Jumah M. A. (2016). Phenotypic and Functional Characterization of Mesenchymal Stem/Multipotent Stromal Cells From Decidua Parietalis of Human Term Placenta. Reprod Sci 23 1193–1207. 10.1177/1933719116632924 - DOI - PubMed
-
- Aghayan H. R., Payab M., Mohamadi-Jahani F., Aghayan S. S., Larijani B., Arjmand B. (2020). GMP-Compliant Production of Human Placenta-Derived Mesenchymal Stem Cells. (New York, NY: Springer; ), 1–13. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

