Bisphenol B Exposure Disrupts Mouse Oocyte Meiotic Maturation in vitro Through Affecting Spindle Assembly and Chromosome Alignment
- PMID: 33392205
- PMCID: PMC7773771
- DOI: 10.3389/fcell.2020.616771
Bisphenol B Exposure Disrupts Mouse Oocyte Meiotic Maturation in vitro Through Affecting Spindle Assembly and Chromosome Alignment
Abstract
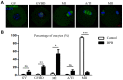
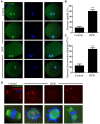
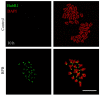
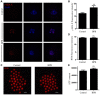
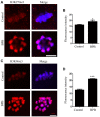
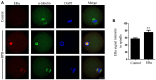
Bisphenol B (BPB), a substitute of bisphenol A (BPA), is widely used in the polycarbonate plastic and resins production. However, BPB proved to be not a safe alternative to BPA, and as an endocrine disruptor, it can harm the health of humans and animals. In the present study, we explored the effects of BPB on mouse oocyte meiotic maturation in vitro. We found that 150 μM of BPB significantly compromised the first polar body extrusion (PBE) and disrupted the cell cycle progression with meiotic arrest. The spindle assembly and chromosome alignment were disordered after BPB exposure, which was further demonstrated by the aberrant localization of p-MAPK. Also, BPB exposure increased the acetylation levels of α-tubulin. As a result, the spindle assemble checkpoint (SAC) was continuously provoked, contributing to meiotic arrest. We further demonstrated that BPB severely induced DNA damage, but the ROS and ATP production were not altered. Furthermore, the epigenetic modifications were changed after BPB exposure, as indicated by increased K3K9me3 and H3K27me3 levels. Besides, the pattern of estrogen receptor α (ERα) dynamics was disrupted with a mass gathering on the spindle in BPB-exposed oocytes. Our collective results indicated that exposure to BPB compromised meiotic maturation and damaged oocyte quality by affecting spindle assembly and chromosome alignment, acetylation of α-tubulin, DNA damage, epigenetic modifications, and ERα dynamics in mouse oocytes.
Keywords: DNA damage; bisphenol B; chromosome alignment; epigenetic modifications; spindle assembly.
Copyright © 2020 Zhang, Ding, Ahmad, Wang, Duan, Miao, Xiong and Huo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Diethylstilbestrol exposure disrupts mouse oocyte meiotic maturation in vitro through affecting spindle assembly and chromosome alignment.Chemosphere. 2020 Jun;249:126182. doi: 10.1016/j.chemosphere.2020.126182. Epub 2020 Feb 11. Chemosphere. 2020. PMID: 32078850
-
Bisphenol F exposure affects mouse oocyte in vitro maturation through inducing oxidative stress and DNA damage.Environ Toxicol. 2022 Jun;37(6):1413-1422. doi: 10.1002/tox.23494. Epub 2022 Feb 26. Environ Toxicol. 2022. PMID: 35218298
-
Bisphenol M inhibits mouse oocyte maturation in vitro by disrupting cytoskeleton architecture and cell cycle processes.Reprod Toxicol. 2024 Oct;129:108667. doi: 10.1016/j.reprotox.2024.108667. Epub 2024 Jul 24. Reprod Toxicol. 2024. PMID: 39059776
-
Carbendazim exposure inhibits mouse oocytes meiotic maturation in vitro by destroying spindle assembly.Food Chem Toxicol. 2023 Sep;179:113966. doi: 10.1016/j.fct.2023.113966. Epub 2023 Jul 26. Food Chem Toxicol. 2023. PMID: 37506866
-
Triphenyltin chloride exposure inhibits meiotic maturation of mouse oocytes by disrupting cytoskeleton assembly and cell cycle progression.Toxicol In Vitro. 2024 Jun;98:105834. doi: 10.1016/j.tiv.2024.105834. Epub 2024 Apr 22. Toxicol In Vitro. 2024. PMID: 38657713
Cited by
-
Azoxystrobin exposure impairs meiotic maturation by disturbing spindle formation in mouse oocytes.Front Cell Dev Biol. 2022 Dec 2;10:1053654. doi: 10.3389/fcell.2022.1053654. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36531942 Free PMC article.
-
Impact of Bisphenol A and its alternatives on oocyte health: a scoping review.Hum Reprod Update. 2024 Dec 1;30(6):653-691. doi: 10.1093/humupd/dmae025. Hum Reprod Update. 2024. PMID: 39277428 Free PMC article.
-
Hexavalent Chromium Disrupts Oocyte Development in Rats by Elevating Oxidative Stress, DNA Double-Strand Breaks, Microtubule Disruption, and Aberrant Segregation of Chromosomes.Int J Mol Sci. 2023 Jun 11;24(12):10003. doi: 10.3390/ijms241210003. Int J Mol Sci. 2023. PMID: 37373153 Free PMC article.
-
The Ovary as a Target Organ for New Generation Bisphenols Toxicity.Toxics. 2025 Feb 26;13(3):164. doi: 10.3390/toxics13030164. Toxics. 2025. PMID: 40137491 Free PMC article. Review.
-
The bisphenol S contamination level observed in human follicular fluid affects the development of porcine oocytes.Front Cell Dev Biol. 2023 Apr 6;11:1145182. doi: 10.3389/fcell.2023.1145182. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37091980 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

